


 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
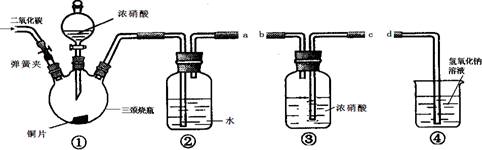
(12��)ij��ѧѧϰС�������ͼ���ṩ������װ�ã��г���������ȥ�����ʵ�飺��֤Ũ����������ԡ�ʵ�����Ũ�����ܽ�NO������NO2���ɴ˵ó��Ľ�����Ũ������������ԡ�
�Իش��й����⣺
��1�������ӿڵ�����˳��Ϊ___________ ____________��
��2������1��������˳��װ��װ�ú���һ���IJ�����___ ____��
����ҩƷ���ɼУ�ͨ��CO2 һ��ʱ�䣬ͨ��CO2��Ŀ����___________________________________________________��
�رյ��ɼУ���װ�â��еĵ���ĩ����������������Һ����Ϊ��_________________ __________________��
��3��װ�âڷ�����Ӧ�Ļ�ѧ����ʽ��___________________________________��
��4����С��ó��Ľ��������ݵ�ʵ��������_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ���˰�У��һ��ѧ������������ѧ�Ծ����������� ���ͣ�ʵ����
(12��)ij��ѧѧϰС�������ͼ���ṩ������װ�ã��г���������ȥ�����ʵ�飺��֤Ũ����������ԡ�ʵ�����Ũ�����ܽ�NO������NO2���ɴ˵ó��Ľ�����Ũ������������ԡ�
�Իش��й����⣺
��1�������ӿڵ�����˳��Ϊ___________ ____________��
��2������1��������˳��װ��װ�ú���һ���IJ�����___ ____��
����ҩƷ���ɼУ�ͨ��CO2һ��ʱ�䣬ͨ��CO2��Ŀ����___________________________________________________��
�رյ��ɼУ���װ�â��еĵ���ĩ����������������Һ����Ϊ��_________________ __________________��
��3��װ�âڷ�����Ӧ�Ļ�ѧ����ʽ��___________________________________��
��4����С��ó��Ľ��������ݵ�ʵ��������_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ���˰�У��һ��ѧ������������ѧ�Ծ��������棩 ���ͣ�ʵ����
(12��)ij��ѧѧϰС�������ͼ���ṩ������װ�ã��г���������ȥ�����ʵ�飺��֤Ũ����������ԡ�ʵ�����Ũ�����ܽ�NO������NO2���ɴ˵ó��Ľ�����Ũ������������ԡ�

�Իش��й����⣺
��1�������ӿڵ�����˳��Ϊ___________ ____________��
��2������1��������˳��װ��װ�ú���һ���IJ�����___ ____��
����ҩƷ���ɼУ�ͨ��CO2 һ��ʱ�䣬ͨ��CO2��Ŀ����___________________________________________________��
�رյ��ɼУ���װ�â��еĵ���ĩ����������������Һ����Ϊ��_________________ __________________��
��3��װ�âڷ�����Ӧ�Ļ�ѧ����ʽ��___________________________________��
��4����С��ó��Ľ��������ݵ�ʵ��������_____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com