
�����仯�������ճ����������Ӧ�ù㷺���Ȼ���������ض��dz�����ˮ����������ͼΪ�Ʊ��Ȼ�������һ�������Ʊ�������صĹ������̡�
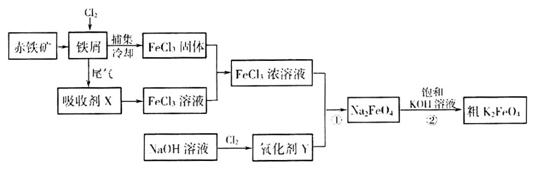 ��ش���������
��ش���������
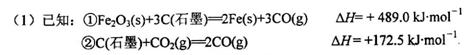
�ó�����Ϊԭ���ڸ�¯���������з�������Ҫ��ӦΪ
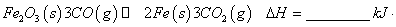 mol
mol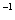 ��
��
(2)���ռ�x������Ϊ____________(д��ѧʽ)��
(3)������YΪ��84����Һ������Ч�ɷ֣����ڼ��������·�Ӧ�ٵ����ӷ���ʽΪ
__________________________________________________________________
(4)���̢�����ij�����½��еģ���Ӧ�Ļ�ѧ����ʽΪ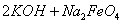 =
=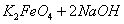 ��˵�����¶���
��˵�����¶���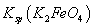 __________
__________ 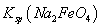 (�>����<��)��
(�>����<��)��
�ٶ��˹�����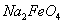 ��ȫת��Ϊ
��ȫת��Ϊ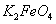 ���������Ƶôֲ�Ʒ
���������Ƶôֲ�Ʒ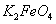 206.25t����Ʒ����Ϊ96������������������Ҫ������Y��������___________t��
206.25t����Ʒ����Ϊ96������������������Ҫ������Y��������___________t��
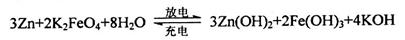 (5)���������һ�����Ͷ��ε�أ����ҺΪǿ����Һ�����ط�ӦΪ��
(5)���������һ�����Ͷ��ε�أ����ҺΪǿ����Һ�����ط�ӦΪ��
�ŵ�ʱ��صĸ�����ӦʽΪ______________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
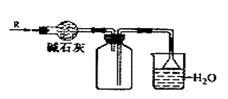
ʵ���ҿ�����ͼ��ʾװ�ø���ռ�ij����R��
�����ն����R����R�� ( )
A.O2 B.HCl C.Cl2 D.NH3
 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ�����ã����������һ����ɫ���壬Ϊ�˽�ͭ�ڿ����еĸ�ʴ�����ij��ѧ��ȤС���ռ�����ͭ���������ɫ�������̽��������������Ϻ������ɫ���ʿ�����ͭ��̼�� �Ρ�
�Ρ�
��С��ͬѧ������ͼװ�ý���ʵ��(���ּг�������)��
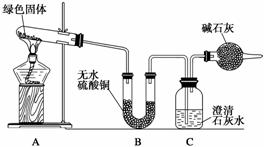
�ٶ��Թ��ڵ���ɫ������м��ȣ�����ȫ�ֽ⣬�۲쵽Aװ������ɫ������ɺ�ɫ��Bװ������ˮ����ͭ�����ɫ��Cװ���г���ʯ��ˮ����ǡ�
��ȡ�������Ⱥ����ɵĺ�ɫ�������Թ��У�����ϡ���ᣬ�۲쵽��ɫ�������ܽ⣬��Һ�����ɫ��
��ȡ����������ɫ��Һ���Թ��У�����һ���ྻ����˿��
�۲쵽��˿�����к�ɫ����������
��ش��������⣺
(1)��ɫ�����к��е�Ԫ����______________________________________________��
(2)���Ⱥ��Թ���ʣ��ĺ�ɫ������________________________________________��
(3)�������ɫ������һ�ִ�������仯ѧʽ������________________________�����ȷֽ�Ļ�ѧ����ʽΪ________________________________________________________
__________________________________________ ______________________________��
______________________________��
(4)����ʵ�鲽����еķ�Ӧ�����ӷ���ʽΪ__________________________________
________________________________________________________________________��
(5)ʵ��װ�����ĸ���ܵ�������_________________________________________��
(6)�����B��C��װ�öԵ����ܷ�ﵽʵ��Ŀ��________(��ܡ����ܡ�)��Ϊʲô��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ֿ�ʼ��Ű�ҹ��ֵ���������SO2����ɿ�����Ⱦ����Ҫԭ�������Ƽ�ѭ�����ɳ�ȥSO2��
(1)�Ƽ�ѭ�����У�����ҺΪNa2SO3��Һ�������շ�Ӧ�����ӷ���ʽ��________________��
(2)��֪H2SO3�ĵ��볣��ΪK1��1.54��10��2��K2��1.02��10��7��H2CO3�ĵ��볣��ΪK1��4.30��10��7��K2��5.60��10��11�������������Թ������ ________________��
A��CO ��HSO
��HSO B��HCO
B��HCO ��HSO
��HSO
C��SO ��HCO
��HCO D��H2SO3��HCO
D��H2SO3��HCO
(3)����Һ����SO2�Ĺ����У�pH��n(SO ):n(HSO
):n(HSO )�仯��ϵ���±���
)�仯��ϵ���±���
| n(SO | 91:9 | 1:1 | 9:91 |
| pH | 8.2 | 7.2 | 6.2 |
���ϱ��ж�NaHSO3��Һ��________________�ԣ���ԭ���ĽǶȽ���ԭ��________________��
����NaHSO3��Һ����Ũ�ȹ�ϵ����ȷ����________(ѡ����ĸ)��
A��c(Na��)��2c(SO )��c(HSO
)��c(HSO )
)
B��c(Na��)>c(HSO )>c(H��)>c(SO
)>c(H��)>c(SO )>c(OH��)
)>c(OH��)
C��c(H2SO3)��c(H��)��c(SO )��c(OH��)
)��c(OH��)
D��c(Na��)��c(H��)��2c(SO )��c(HSO
)��c(HSO )��c(OH��)
)��c(OH��)
(4)������Һ��pH����ԼΪ6ʱ����������������������ʾ��ͼ���£�
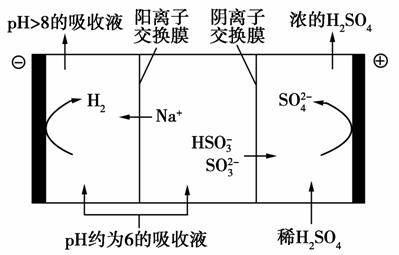
������Һ���������е��ܷ�Ӧ����ʽ��________________��
�ڵ��缫����1 mol����ת��ʱ��������Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������е�A��B��C��D��E����Ԫ�أ�ԭ��������������A��D��C��E�ֱ�ͬ���壬AΪ�ǽ���Ԫ�أ���A��B��ԭ������֮�͵���C��ԭ��������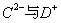 �ĺ����������ȡ�������˵����ȷ����
�ĺ����������ȡ�������˵����ȷ����
A��B��Aֻ�����BA3������
B��C��D��E�γɵĻ�����ˮ��Һ�����Լ���
C��A��B��C�γɵĻ�����һ�����ܷ���ˮ�ⷴӦ
D��E���������Ӧ��ˮ����ֻ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ܵ������
�ٹ���ʳ�� �ڰ�ˮ �������ܲ�˿������ ���Ȼ����Һ��������ˮ��Һ
A���٢ܢޢ� B���ݢޢ� C���ܢ� D���ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ba(OH)2��Һ����������Һ�У�ʹSO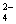 ȫ��ת����BaSO4��������ʱ��Ԫ��
ȫ��ת����BaSO4��������ʱ��Ԫ��
����Ҫ������ʽ��
 A��Al3+ B��Al(OH)3 C��AlO2�� D��Al3+ ��Al(OH)3
A��Al3+ B��Al(OH)3 C��AlO2�� D��Al3+ ��Al(OH)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijһ��ɫ����Һ��ֻ���ܴ���H+��Ba2+��Mg2+��Cu2+��OH-��HCO3-��
CO32-��NO3-��SO42- �е�һ�ֻ��֡���֪����Һ����Al��Ӧ�ų�H2
��1������Ӧ����Al3+����ԭ��Һ��һ������ ���ܴ��ڵ�������
��2������Ӧ����AlO2- ����ԭ��Һ��һ������ ���ܴ��ڵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E���������о�����ͬһ�ַǽ���Ԫ�أ������ܷ�����ͼ��ʾ��ת����ϵ����Ԫ��(��R��ʾ)�ĵ�������NaOH��Һ��Ӧ�� ����(Na2RO3)��������
����(Na2RO3)��������
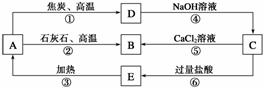
��ش��������⣺
(1)д�������ʵĻ�ѧʽ��A__________��B__________��C__________��D__________��E__________��
(2)д����Ӧ�ٵĻ�ѧ����ʽ��____________________________________________��
�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________��
(3)д����Ӧ�ܵ����ӷ���ʽ��__________________________________________��
(4)д����Ӧ�ݵ����ӷ���ʽ��___________________________________________��
(5)H2CO3������ǿ��E�ģ��������ӷ���ʽ����֤����
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com