
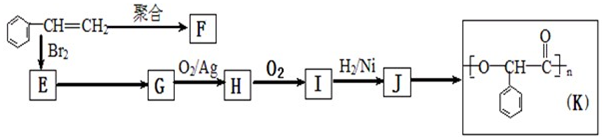
�ɱ���ϩ�����з�Ӧ���Ƶ�F��K���ָ߷��ӻ�������Ƕ��dz��õ����ϡ�
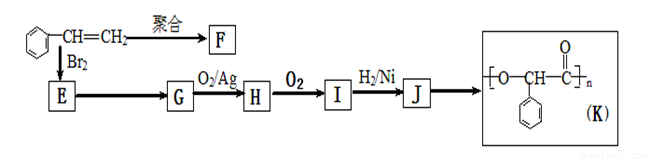
��1��J�����������ŵ�����Ϊ ��K�����������ŵĽṹ��ʽΪ
��2���ۺ���F�Ľṹ��ʽ�� ��I�ķ���ʽ�� ��
��3��Eת��ΪG�Ļ�ѧ����ʽ�� ����Ӧ��������
��4����һ�������£�������J����ȥ������ˮ�γ�һ����Ԫ��״�����д���û�����Ľṹ��ʽ ��
��5��д��J ��һ�ַ�������������ͬ���칹��X���ʽ ��
��1mol X������3mol NaOH��Ӧ ��X��������ԭ�Ӻ˴Ź���������4���壬
��1���ǻ����Ȼ� ����1�֣���
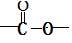 ��1�֣�
��1�֣�
��2��
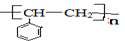 ��2�֣�
C8H6O3 ��2�֣�
��2�֣�
C8H6O3 ��2�֣�
��3��
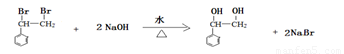 ��2�֣�ȡ����Ӧ��1�֣�
��2�֣�ȡ����Ӧ��1�֣�
��4��
 ��2�֣�
��2�֣�
��5��
 ��3�֣�
��3�֣�
��������
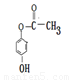
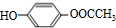
�����������1����K�Ľṹ��ʽ���ж�J�к����ǻ����Ȼ���KΪ�߾�����������Ϊ��������2��FΪ����ϩ�ļӾ۲��������֧����IΪ���ʻ������ᣬ����ʽΪC8H6O3����3��E��GΪ±������ˮ�ⷴӦ������ΪNaOH��ˮ��Һ���ǻ�ȡ������ԭ�ӡ���4��J�к���һ���Ȼ���һ���ǻ���������ͨ���γ����������ɻ�������5���ɢ�֪X�к���һ�����ǻ�����һ��Ϊ���������ɷ��������γɵģ�����OH����OOCCH3���ɢ�֪����ȡ�������ڶ�λ��
���㣺�����л���ĺϳɣ����鿼���ۺϷ��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


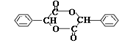
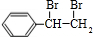 +2NaOH
+2NaOH| ˮ |
| �� |
 +2NaBr
+2NaBr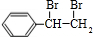 +2NaOH
+2NaOH| ˮ |
| �� |
 +2NaBr
+2NaBr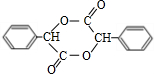


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com