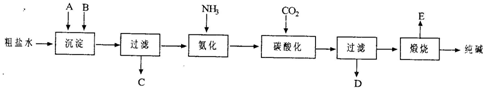
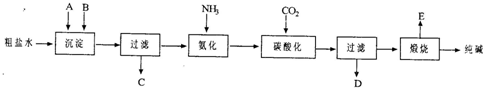
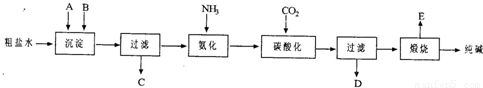
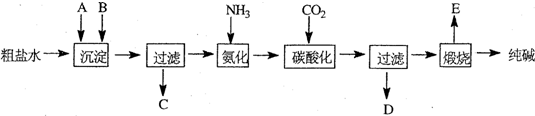
��ҵ��������Ĺ�������ʾ��ͼ���£�
���������գ�
��1������ˮ���������A��B�����ʣ�������A��Դ��ʯ��Ҥ������д��A��B�Ļ�ѧʽ��
A
��B
��
��2��̼�ữ������Ӧ�Ļ�ѧ����ʽ��
��
��3����ĸҺ
��ѡ��ͼ����ĸ����ͨ����������ϸСʳ�ο�������ȴ��������Ʒ��ͨ������������
��
A������NH
4+��Ũ�ȣ�ʹNH
4Cl���������
B��ʹNaHCO
3���������
C��ʹNaHCO
3ת��ΪNa
2CO
3�����������NH
4Cl����
��4�����������Լ�����鸱��ƷNH
4Cl�Ƿ��ķ�����������
��
��5��Xg�����Ʒ������̼�����ƣ���ּ��ȷֽ������������Yg������Ʒ��̼�����Ƶ����������ɱ�ʾΪ
��
��6�������������ƺͽ�̿��ʯ��ʯ�ڸ����½������գ��ٽ�ȡ���ᾧ���Ƶô����Ӧ�Ļ�ѧ����ʽΪ
����֪����֮һΪCaS����




 ����5��2���ϵ�д�
����5��2���ϵ�д�