
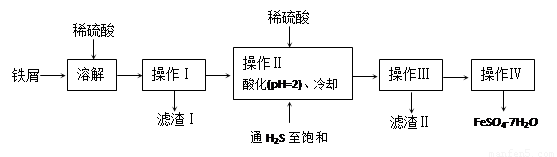
�̷���FeSO4��7H2O��������ȱ����ƶѪҩƷ����Ҫ�ɷ֡���������������м�����������������������ʣ�Ϊԭ�����������̷���һ�ַ�����
��֪�������±���H2S��Һ��pHԼΪ3.9��SnS������ȫʱ��Һ��pHΪ1.6��FeS��ʼ����ʱ��Һ��pHΪ3.0��������ȫʱ��pHΪ5.5��
��1������II�У�ͨ�����������͵�Ŀ���� _________________________ ������Һ���������ữ��pH��2��Ŀ���� _______________________________ ��
��2�������Ƶõ��̷��������Ƿ���Fe3+��ʵ������� ��
��3���ⶨ�̷���Ʒ��Fe2+�����ķ����ǣ�
a.��ȡ3.7200g�̷���Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
����֪KMnO4������Һ��Fe2+��Ӧʱ����ԭΪMn2+����д���÷�Ӧ���� ��
���ڵζ�ʵ���в���ѡ�� ʽ�ζ��ܣ������� ��
�ۼ���������Ʒ��FeSO4��7H2O����������Ϊ ����С����ʾ��������λС������
��1���ٳ�ȥ��Һ�е�Sn2+������ֹ�������ӱ���������ֹ�����������ɳ���
��2��ȡ������������ˮ���μ�KSCN��Һ������Һ���Ժ�ɫ��������Һ�����������ӡ���Һ�Ժ�ɫ������Һ�к��������ӣ�2�֣�
��3����5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��2�֣�
�ڼ2�֣������������ǿ�����������ḯʴ�齺�ܣ�2�֣� ��0.75��3�֣�
��������
�����������1�����������ǿ��ԭ�ԣ����Է�ֹ�������ӱ�����������Ϊ�����±���H2S��Һ��pHԼΪ3.9����SnS������ȫʱ��Һ��pHΪ1.6�����Բ���II�У�ͨ�����������͵���һ��Ŀ���dz�ȥ��Һ�е�Sn2+������FeS��ʼ����ʱ��Һ��pHΪ3.0��������ȫʱ��pHΪ5.5����˲���������Һ���������ữ��pH=2��Ŀ���Ƿ�ֹ�����������ɳ�����
��2�����������̷��������Ƿ���Fe3+��ʵ�����������������������KSCN��Һ������ɫ��Ӧʹ��Һ�Ժ�ɫ�����жϡ���ȡ������������ˮ���μ�KSCN��Һ������Һ���Ժ�ɫ��������Һ�����������ӡ���Һ�Ժ�ɫ������Һ�к��������ӡ�
��3���ٸ��������Һ����ǿ�����ԣ��������������ӡ���Ӧ��MnԪ�صĻ��ϼ۴ӣ�7�۽��͵���2�ۣ��õ�2�����ӡ����������ڷ�Ӧ��ʧȥ1�����ӣ���˸��ݵ��ӵ�ʧ�غ��֪����������������뻹ԭ���������ӵ����ʵ���֮�ȣ�1:5�����Է�Ӧ�����ӷ���ʽΪ5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��
�����ڸ��������Һ����ǿ�����ԣ��������ܣ��Ӷ���ʴ�齺�ܣ����Բ����ü�ʽ�ζ��ܣ�Ӧ������ʽ�ζ��ܡ�
���������ữ��0.01000mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL�������ĸ�����ص����ʵ�����0.01000mol/L��0.0200L��0.0002mol
5Fe2++8H++MnO4-��5Fe3++Mn2++4H2O
5mo 1mol
n(Fe2+) 0.0002mol
����õ�n(Fe2+)��0.001mol
��250mL��Һ�к�n(Fe2+)��0.001mol��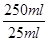 ��0.01mol
��0.01mol
����FeSO4•7H2O���ʵ���Ϊ0.01mol
������0.01mol��278g/mol��2.78g
������������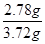 ��0.75
��0.75
���㣺�����������������Ʊ�ʵ��ķ�����������ۣ�������ԭ�ζ����й��ж�������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| O | 2- 4 |
| O | 2- 7 |
| O | 2- 7 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com