
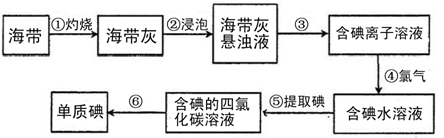
ŗ£“ųÖŠŗ¬ÓŠ·įø»µÄµā£®ĪŖĮĖ“Óŗ£“ųÖŠĢįČ”µā£¬Ä³ŃŠ¾æŠŌѧĻ°Š”×éÉč¼Ę²¢½ųŠŠĮĖŅŌĻĀŹµŃé£ŗ


ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)²½Öč¢Ł×ĘÉÕŗ£“ųŹ±£¬³żŠčŅŖČż½Å¼ÜĶā£¬»¹ŠčŅŖÓƵ½µÄŹµŃéŅĒĘ÷ŹĒ______________(“ÓĻĀĮŠŅĒĘ÷ÖŠŃ”³öĖłŠčµÄŅĒĘ÷£¬ÓƱźŗÅ×ÖÄøĢīŠ“ŌŚæհד¦)£®
A£®ÉÕ±””””B£®ŪįŪö””””C£®±ķĆęĆó””””D£®ÄąČż½Ē””””E£®¾Ę¾«µĘ””””F£®øÉŌļĘ÷
(2)²½Öč¢ŪµÄŹµŃé²Ł×÷Ćū³ĘŹĒ______________£»²½Öč¢ŽµÄÄæµÄŹĒ“Óŗ¬µā±½ČÜŅŗÖŠ·ÖĄė³öµ„ÖŹµāŗĶ»ŲŹÕ±½£¬øĆ²½ÖčµÄŹµŃé²Ł×÷Ćū³ĘŹĒ_____________________________________________£®
(3)²½Öč¢Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ___________________________________________________£®
(4)²½Öč¢ŻÖŠ£¬Ä³Ń§ÉśŃ”ŌńÓƱ½Ą“ĢįČ”µāµÄĄķÓÉŹĒ___________________________________£®
(5)ĒėÉč¼ĘŅ»ÖÖ¼ģŃéĢįČ”µāŗóµÄĖ®ČÜŅŗÖŠŹĒ·ń»¹ŗ¬ÓŠµ„ÖŹµāµÄ¼ņµ„·½·Ø£ŗ_______________£®
””””(1)BDE(2·Ö£¬²»Č«øų1·Ö£¬“ķŃ”²»øų·Ö)
””””(2)¹żĀĖ””ÕōĮó(ø÷1·Ö)
””””(3)2I££«MnO2£«4H+£½Mn2+£«I2£«2H2O(2·Ö)
””””(4)±½ÓėĖ®»„²»ĻąČÜ£»µāŌŚ±½ÖŠµÄČܽā¶Č±ČŌŚĖ®ÖŠ“ó(2·Ö)£®
””””(5)ȔɣĮæĢįČ”µāŗóµÄĖ®ČÜŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČė¼øµĪµķ·ŪŹŌŅŗ£»¹Ū²ģŹĒ·ń³öĻÖĄ¶É«(Čē¹ū±äĄ¶£¬ĖµĆ÷»¹ÓŠµ„ÖŹµā)(2·Ö)


 ŠĀæĪ±ź½×ĢŻŌĶĮѵĮ·ĻµĮŠ“š°ø
ŠĀæĪ±ź½×ĢŻŌĶĮѵĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
×° ÖĆ |
 |
 |
 |
| ĻÖ Ļó | ½šŹōA²»¶ĻČܽā | CµÄÖŹĮæŌö¼Ó | AÉĻÓŠĘųĢå²śÉś |
| Õż¼«·“Ó¦Ź½ |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| b |
| a(1-m) |
| 100b |
| a(1-m%) |
| b |
| a(1-m) |
| 100b |
| a(1-m%) |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com