
| |||||||||||||||
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
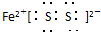
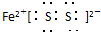
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
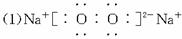
��1��д��������B�ĵ���ʽ_____________________��
��2����Ӧ�ܵ����ӷ���ʽΪ_____________________��
��Ӧ�ݵĻ�ѧ����ʽΪ___________________________________________________��
��3����֪ÿ����16 g E���ų�106.5 kJ����������Ӧ�ٵ��Ȼ�ѧ����ʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
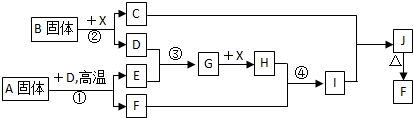
�������п�ͼ��ϵ��գ���֪��Ӧ�١����ǹ�ҵ�����е���Ҫ��Ӧ��D��E������Ϊ���塢X������Ϊ��ɫҺ�壬H��E��Է�������֮��Ĺ�ϵΪ��Mr(H) ��Mr(E) =34����֪C��ɫ��Ӧ����ʻ�ɫ��
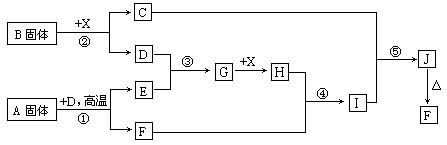
(1) ��Ӧ�ڵĻ�ѧ����ʽ��__________________________________��
����C�ĵ���ʽ��_______________________��
(1)������A���������Ļ�ѧ���У�_______________________��
(2)��Ӧ�ܵ����ӷ���ʽ��_______________________________��
��Ӧ�ݵĻ�ѧ����ʽ��__________________________________��
(3)��֪ÿ����16g E���ų�106.5 kJ��������Ӧ�ٵ��Ȼ�ѧ����ʽΪ��
_________________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣��������п�ͼ��ϵ��ա���֪������XΪ��ɫҺ�壬D��E��Ϊ��ɫ���壬
G��һ����Ҫ�Ĺ�ҵ��Ʒ��C����ɫ��ӦΪ��ɫ��E��F����Է�����֮��Ĺ�ϵΪ
M��F��=M��E��+16���ش��������⣺
��1��A�л�ѧ��������Ϊ ��д��B��һ����; ��
��2��������A����FeCl3��Һ�е���Ҫ������ ��
��3��д������B��ˮ��Һ��CuSO4��Һ��Ӧ�����ӷ���ʽ ��
��4��д����Ӧ�ڵĻ�ѧ����ʽ ��
��5�����ⶨ����Ӧ����ÿ����1.0gX���ų�3.2KJ�������������£�����д����Ӧ��
���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�����и�����ѧ���ܲ⻯ѧ�Ծ��������棩 ���ͣ������
(12��)�������п�ͼ��ϵ��ա���֪��Ӧ�١����ǹ�ҵ�����е���Ҫ��Ӧ��D��E������Ϊ���塢X������Ϊ��ɫҺ�壬H��E��Է�������֮��Ĺ�ϵΪ��Mr(H)-Mr(E)=34����֪C����ɫ��Ӧ�ʻ�ɫ��
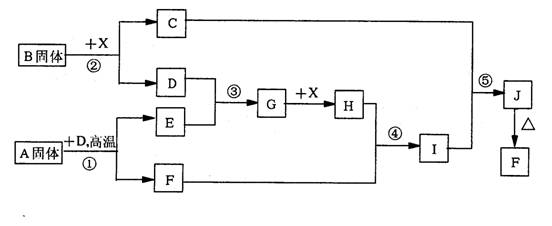
(1)������B���������Ļ�ѧ����___________________
(2)��Ӧ�ܵ����ӷ���ʽ��________________________________
��Ӧ�ݵĻ�ѧ����ʽ��________________________________
(3)��֪ÿ����16gE���ų�106.5kJ��������Ӧ�ٵ��Ȼ�ѧ����ʽΪ��
______________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com