
 ��
��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO2������ʹƷ������Ը��������Һ��ɫ����ԭ����ͬ |
| B�������ɽ�NO2 ת�����������ʣ�����Ϊ�������л�ԭ�� |
| C��NH4NO3��Һ��HNO3��Һ�еμ�ʯ�ﶼ���ɫ������Ϊ���Ǿ��ܵ����H+ |
| D��pH=3��������pH=11��MOH��Һ�������Ϻ���Һ�ʼ��ԣ�˵��MOHΪ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �������� | ��ʼ�γ��������������pH | ��ȫ�γ��������������pH |
| Fe2+ | 7.0 | 9.0 |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
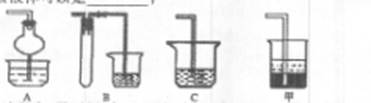
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
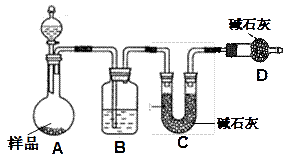
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
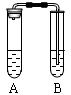
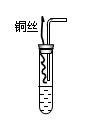
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
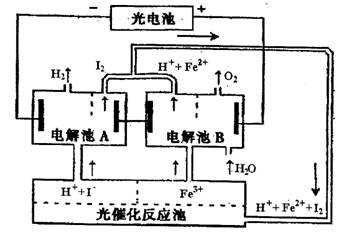
|
| A����Ƹ�ѭ��ϵͳ��Ŀ������ȡI2 | B������A�����ӷ�Ӧ����ʽ�� |
C�������Ӧ�������ӷ�Ӧ����ʽΪ�� | |
| D����ϵͳ�е�Fe3+��H2O��I-����ѭ��ʹ�õ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ȡNa2CO3��Һ������ȡ�����࣬Ϊ�˲��˷ѣ��ְѹ������Լ������Լ�ƿ�С� |
| B����ʣ��Ľ����ƷŻ�ԭʢ�Ž����Ƶ��Լ�ƿ�С� |
| C������������ʹNaCl ����Һ������ʱ��Ӧ����������NaCl ��Һȫ�����ɲ�ֹͣ���ȡ� |
| D����Ũ��������һ�����ʵ���Ũ�ȵ�ϡ����ʱ��Ũ��������ˮ��Ӧ��ȴ�����²���ת�Ƶ�����ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ü��ȵķ��������Ȼ��ƺ��Ȼ�淋Ĺ������� |
| B����ˮ������ȩ������������ |
| C���ú˴Ź���������δ֪��C2H6O�ķ��ӽṹ |
| D����10mL��Ͳ��ȡ5.00mL1.00 mol��L-1��������0.100mol��L-1���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com