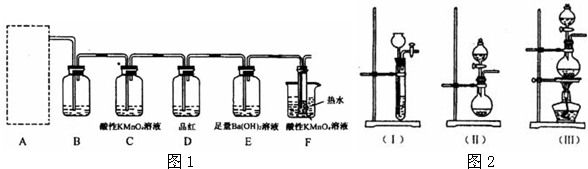
ijѧ���������ͼ��ʾ��װ�ý���ʵ�飬��װ������װ�Լ���ʵ�������ʵ���������£�
��װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң���C��D�ж�װŨ�����Eƿ��װ���Լ�Y
��ʵ������������ǣ���ˮԡ����Aƿ����D��Ũ���Ỻ������E�����Լ�Y���ã�����C�е����д������ݷų���Aƿ��X��ɫ����B�ܻӷ���������ɵ�ȼ��
��ش��������⣺
��1��Eƿ����װ���Լ�Y��
c
c
����д��ţ�
a������ʳ��ˮ��b��MnO
2��NaCl�Ļ���c��Ũ����
��2��C��ŨH
2SO
4�����������
����HCl����
����HCl����
��Dƿ��ŨH
2SO
4�����������
����Ũ�����л��е�ˮ�֣�ŨH2SO4����ˮ���ȣ�������HCl�����ݳ�
����Ũ�����л��е�ˮ�֣�ŨH2SO4����ˮ���ȣ�������HCl�����ݳ�
��
��3��Aƿ�з�����Ӧ�Ļ�ѧ����ʽ��
����Ӧ������
ȡ����Ӧ
ȡ����Ӧ
�������ɵ�
������
������
��д���ƣ���B���ڴ���ȼ��
��4����ˮ��X��ѡ��
��ˮCuSO4
��ˮCuSO4
��������ָʾ�����õ�ԭ����
ʵ������й۲쵽��ˮCuSO4�ɰױ�����˵����Ӧ����ˮ���ɣ���CuSO4�������CuSO4?5H2O
ʵ������й۲쵽��ˮCuSO4�ɰױ�����˵����Ӧ����ˮ���ɣ���CuSO4�������CuSO4?5H2O
��
��5����ʵ����֤���Ҵ������к�����ԭ�ӵ�������
��ˮCuSO4����֤���˷�Ӧ��һ����ˮ���ɣ�ˮ�е���Ԫ�ز���������HCl����ֻ�����Ҵ��ṩ
��ˮCuSO4����֤���˷�Ӧ��һ����ˮ���ɣ�ˮ�е���Ԫ�ز���������HCl����ֻ�����Ҵ��ṩ
��
��6�������װ���е�Cƿȥ����ʵ��Ŀ���Ƿ��ܹ��ﵽ��
����
����
����ܡ����ܡ�������Ϊ
HCl�ӷ�ʱ����ˮ������������ȥ�����ж�ʹ��ˮCuSO4������ˮ�Ƿ��������Ҵ�
HCl�ӷ�ʱ����ˮ������������ȥ�����ж�ʹ��ˮCuSO4������ˮ�Ƿ��������Ҵ�
��

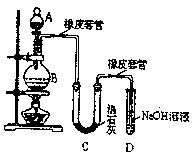
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
 ijѧ���������ͼ��ʾ��װ�ý���ʵ�飬��װ������װ�Լ���ʵ�������ʵ���������£�
ijѧ���������ͼ��ʾ��װ�ý���ʵ�飬��װ������װ�Լ���ʵ�������ʵ���������£�