
 �ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�
�ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�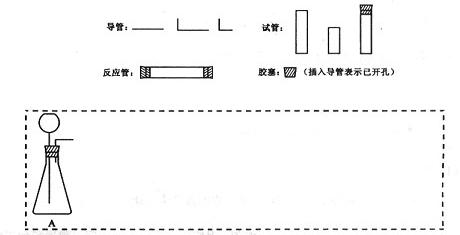
| �������� | �������������� | ���� |
| A | ʯ��ʯ��ϡ���� | ʯ��ʯ����������CO2 |
| | | |
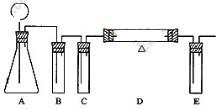
| ������� | �������������� | ���� |
| B | ����̼��������Һ | ��ȥCO2�е�HCl���� |
| C | Ũ���� | ��ȥCO2�е�ˮ�� |
| D | ����ľ̿�� | ��CO2��Ӧ����CO |
| E | ����ʯ��ˮ | ����δ��Ӧ��CO2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ���� | ���������Һ�����mL�� | �ζ���ʼ������mL�� | �ζ�����������mL�� |
| �� | 25��00 | 0��00 | 10.00 m |
| �� | 25��00 | 1��00 | 11.02 |
| �� | 25��00 | 0��22 | 12.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| | ����ʽ | ��ɫ��״̬ | ˮ���� | �۵�/�� | �ܶ�/g��cm��3 |
| ���ᾧ�� | H2C2O4��2H2O | ��ɫ���� | ������ˮ | 101.5 | 1.650 |
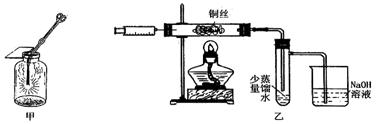
 ��2���������ɺ������Թ��е�����ˮ��Ϊ����ɫ����ʱ��������������ʹ����NaOH��Һ�������Թ��У������Թ����� ɫ�ij����������÷�Ӧ���뷽��ʽΪ ��
��2���������ɺ������Թ��е�����ˮ��Ϊ����ɫ����ʱ��������������ʹ����NaOH��Һ�������Թ��У������Թ����� ɫ�ij����������÷�Ӧ���뷽��ʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | ʵ����� | Ԥ������ |
| ����һ��FeԪ��ֻ��______�� | ����������Һ�е���KSCN��Һ ����ϡ����KMnO4��Һ�е���������Һ | KSCN��Һ���������� |
| �����;FeԪ��ֻ��______�� | ϡ����KMnO4��Һ��ɫ_____ | |
| ��������FeԪ�ؼ���+2������+3�� | KSCN��Һ��______ɫ ϡ����KMnO4��Һ��ɫ______ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��CO
��CO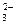 ��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش�
��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
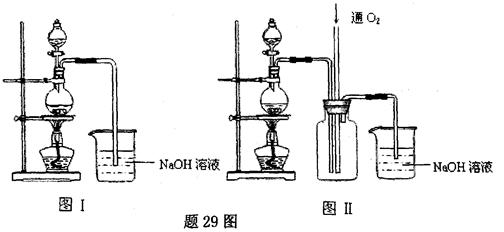
 �����нᾧˮ�ĺ���ʱ������й��������±���
�����нᾧˮ�ĺ���ʱ������й��������±���| ����ǰ���� | ���Ⱥ����� | |
| m1�������� | m2������+���壩 | m3������+��ˮCuSO4�� |
| 5.4g | 7.9g | 6.8g |
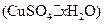 �нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�нᾧˮ������ʵ���У������������ٽ��� �Ρ�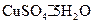 ���
��� �� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
�� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������Ȼ�þ��ȡ����þ |
| B�������ƽᾧˮ�������ʯ�ҹ�����ȡ���顣 |
| C��Ũ�������廯�ƹ�����ȡ�廯�� |
| D������̼������Һ��ȥ������̼�е��Ȼ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com