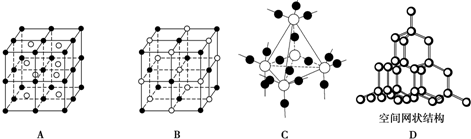
��2011?��ͬģ�⣩A��B��C��D��E����ѧ������5�ֻ����A��B�������Ԫ��X��Y�ĵ����������г����Ľ�����������ʼ�Ĺ�ϵ��ͼ��ʾ��

��ش��������⣺
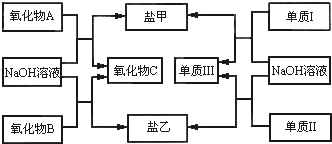
��1��X�ĵ�����A��Ӧ�Ļ�ѧ����ʽ��
��
��2�����Լ�1��NaOH��Һ��X�ĵ������Լ�1��Ӧ�����ӷ���ʽ��
2Al+2H2O+2OH-�T2AlO2-+3H2��
2Al+2H2O+2OH-�T2AlO2-+3H2��
��
��3�����Լ�1���Լ�2����ϡ���ᣮ
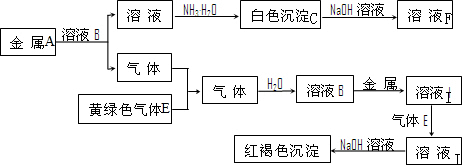
�ټ�������D����Һ�н������ӵķ�����
ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ���ɫ����֤��ԭ��Һ�к���Fe3+
ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ���ɫ����֤��ԭ��Һ�к���Fe3+
��
�ڽ�����C����ˮ������Һ�����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��
Al
3++3H
2O

Al��OH��
3+3H
+Al
3++3H
2O

Al��OH��
3+3H
+��
��ij��Ч��ˮ������Y��OH��S0
4�ۺϵõ��ģ���ҵ����E��ϡ�������������Ϊԭ�����Ʊ�Y��OH��SO
4����Ӧ����NO���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��
2FeSO4+2NaNO2+H2SO4�T2Fe��OH��SO4+Na2SO4+2NO��
2FeSO4+2NaNO2+H2SO4�T2Fe��OH��SO4+Na2SO4+2NO��
��



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

 Al��OH��3+3H+
Al��OH��3+3H+ Al��OH��3+3H+
Al��OH��3+3H+