
��M��N������Һ�����ⶨ��������Һ�к�������12�����ӣ�Al3����Cl����Na����K����NO ��OH����Fe2����AlO
��OH����Fe2����AlO ��CO
��CO ��NH
��NH ��SO
��SO ��H����
��H����
(1)������б�����ʵ��ٵĽ��ۺ�ʵ��ڵ�ʵ�������Լ�����
| ʵ�������Լ����� | ���� |
| ��ȡ����N��Һ�μ����������ᱵ��Һ���������� | |
| �� | ȷ��M��Һ�к���Na��������K�� |
| ����pH��ֽ���M��Һ��pH��ֽ����ɫ |
(2)����(1)�е�ʵ��ش�
NO ������________��Һ�У�������____________________________________________��
������________��Һ�У�������____________________________________________��
Cl��������________��Һ�У�������________________________________��
(3)����(1)�е�ʵ��ȷ����M��Һ�к��е�����Ϊ________________________________��
�𰸡�(1)��N��Һ�в���CO ��SO
��SO (��M��Һ��һ������CO
(��M��Һ��һ������CO ��SO
��SO )
)
��ȡM��Һ������ɫ��Ӧ����ɫΪ��ɫ��������ɫ�ܲ����۲������ɫ��������ɫ
(2)M��N��Һ�к���H����Fe2����Al3����NH ��K��������N��ҺΪ���ԣ��ֺ���Fe2��������N��Һ�в���NO
��K��������N��ҺΪ���ԣ��ֺ���Fe2��������N��Һ�в���NO
N��������Һ�ʵ�����ԭ����ȷ��Cl��������N��Һ��
(3)OH����AlO ��CO
��CO ��SO
��SO ��Na����NO
��Na����NO
����������N��Һ�еμ����������ᱵ��Һ������������˵��N��Һ�в���CO ��SO
��SO ����ôM��Һ��һ������CO
����ôM��Һ��һ������CO ��SO
��SO ��ȡM��Һ������ɫ��Ӧ����ɫΪ��ɫ��֤������Na����������ɫ�ܲ����۲������ɫ��������ɫ��˵��M�в���K������pH��ֽ���M��Һ��pH��ֽ����ɫ��˵��M��Һ�Լ��ԣ����д�����OH������ôN��Һ�к��д�����H����AlO
��ȡM��Һ������ɫ��Ӧ����ɫΪ��ɫ��֤������Na����������ɫ�ܲ����۲������ɫ��������ɫ��˵��M�в���K������pH��ֽ���M��Һ��pH��ֽ����ɫ��˵��M��Һ�Լ��ԣ����д�����OH������ôN��Һ�к��д�����H����AlO �����ܴ�����������Һ�У�Al3����Fe2����NH
�����ܴ�����������Һ�У�Al3����Fe2����NH �����ܴ����ڼ�����Һ�У������ж�M��Һ����OH����AlO
�����ܴ����ڼ�����Һ�У������ж�M��Һ����OH����AlO ��CO
��CO ��SO
��SO ��Na����N��Һ�к���H����Fe2����Al3����NH
��Na����N��Һ�к���H����Fe2����Al3����NH ��K��������N��ҺΪ���ԣ��ֺ���Fe2��������N��Һ�в���NO
��K��������N��ҺΪ���ԣ��ֺ���Fe2��������N��Һ�в���NO ��������Һ�ʵ�����ԭ����ȷ��Cl��������N��Һ�С�
��������Һ�ʵ�����ԭ����ȷ��Cl��������N��Һ�С�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���������Һ�з������·�Ӧ��
��16H����10Z����2XO ===2X2����5Z2��8H2O
===2X2����5Z2��8H2O
��2A2����B2===2A3����2B��
��2B����Z2===B2��2Z��
�ɴ��ж�����˵����ȷ����(����)
A����ӦZ2��2A2��===2A3����2Z�����ܽ���
B��ZԪ���ڢ٢۷�Ӧ�о�������
C��������������ǿ��˳����XO ��Z2��B2��A3��
��Z2��B2��A3��
D����ԭ����ǿ������˳����A2����B����Z����X2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
5.6 g Cu��Mg�Ͻ���һ����������ǡ����ȫ��Ӧ���ռ���NO��NO2�Ļ������V L(��״��)����Ӧ�����Һ�м�������NaOH��Һ��������ȫ������ˡ�ϴ�ӡ�����Ƶ�����Ϊ10.7 g����V����(����)
A��2.24 B��4.48
C��6.72 D��7.84
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ij��Һ�еμ���ˮ���ټ���KSCN��Һ����Һ��Ѫ��ɫ������Һ��һ������Fe2�������ж��Ƿ���ȷ��Ϊʲô��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijδ֪��Һ���ܺ�Cl����CO ��Na����SO
��Na����SO ��Al3��������Һ������ɫʯ����ֽ�ϣ���ֽ��졣ȡ������Һ���μ������ữ���Ȼ�����Һ���а�ɫ�������ɣ����ϲ���Һ�еμ���������Һ��������ɫ�����������жϺ�������(����)
��Al3��������Һ������ɫʯ����ֽ�ϣ���ֽ��졣ȡ������Һ���μ������ữ���Ȼ�����Һ���а�ɫ�������ɣ����ϲ���Һ�еμ���������Һ��������ɫ�����������жϺ�������(����)
A��һ����Cl�� B��һ����SO
C��һ��û��Al3�� D��������CO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ���ܺ���Cl����SO ��CO
��CO ��NH
��NH ��Fe3����Al3����K����ȡ����Һ100 mL���������NaOH��Һ�����ȣ��õ�0.02 mol���壬ͬʱ�������ɫ���������ˣ�ϴ�ӣ����գ��õ�1.6 g���壻��������Һ�м�����BaCl2��Һ���õ�4.66 g����������ij������ɴ˿�֪ԭ��Һ�� (����)
��Fe3����Al3����K����ȡ����Һ100 mL���������NaOH��Һ�����ȣ��õ�0.02 mol���壬ͬʱ�������ɫ���������ˣ�ϴ�ӣ����գ��õ�1.6 g���壻��������Һ�м�����BaCl2��Һ���õ�4.66 g����������ij������ɴ˿�֪ԭ��Һ�� (����)
A�����ٴ���5������
B��Cl��һ�����ڣ���c(Cl��)��0.4 mol·L��1
C��SO ��NH
��NH һ�����ڣ�Cl�����ܲ�����
һ�����ڣ�Cl�����ܲ�����
D��CO ��Al3��һ�������ڣ�K�����ܴ���
��Al3��һ�������ڣ�K�����ܴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����е������ʵĵ��뷽��ʽ
(1)H2SO4______________________________________________________________________��
(2)H2CO3______________________________________________________________________��
(3)Ca(OH)2____________________________________________________________________��
(4)Fe(OH)3_____________________________________________________________________��
(5)NH3·H2O____________________________________________________________________��
(6)NaCl_______________________________________________________________________��
(7)BaSO4_____________________________________________________________________��
(8)NaHSO4_____________________________________________________________________��
(9)NaHCO3____________________________________________________________________��
(10)NaHSO4(����)_____________________________________________________________��
(11)Al2O3(����)___________________________________________________________��
(12)CH3COOH_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ھ����˵����һ����ȷ���� (����)��
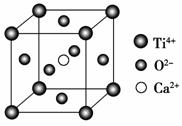
CaTiO3�ľ���ṹģ��(ͼ��Ca2����O2����
Ti4���ֱ�λ������������ġ����ĺͶ���)
A�����Ӿ����ж����ڹ��ۼ�
B������ͼ��CaTiO3������ÿ��Ti4����12��O2�������
C��SiO2������ÿ����ԭ����������ԭ���Թ��ۼ�����
D������������۵㶼�ȷ��Ӿ�����۵��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com