
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O�� CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��| 0.748g |
| 1g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
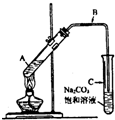 ��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о��� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O| ����� | ���� | ��Ӧ���� | C�б���̼������Һ������߶� |
| �� | 2mL98%Ũ���� | 20��ʱ��Һ������ɫ���淴Ӧ���У���Һ��ɫ������ɺ�ɫ�����������ݣ� | 2.10cm |
| �� | 2mL14mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� | 2.14cm |
| �� | 2mL10mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� | 2.16cm |
| �� | 2mL7mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ�����ݣ� | 2.00cm |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ���������и������ĴΣ�12�£��¿���ѧ�Ծ����������� ���ͣ������
��12�֣�ij��ȤС��Ϊ�о�������������ȡ����������ʵ�顣
ʵ��һ ���Ҵ���������ȡ��������
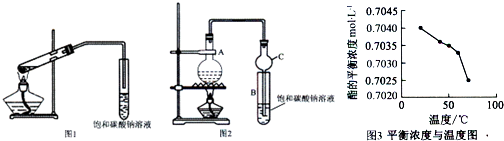
�ס���ͬѧ�ֱ�ʹ��ͼ1��ͼ2װ����ȡ����������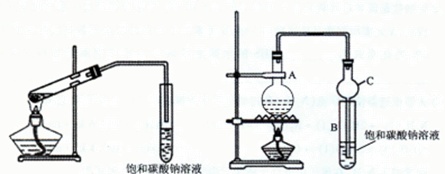
ͼ1 ͼ2
��1����ȡ���������Ļ�ѧ����ʽΪ
��2��������̼������Һ��������������Һ�Ƿ���У�������ԭ��
��3��ͼ1����ĩ�˲��ܲ��뱥��̼������Һ����ͼ2װ���е�����C��ĩ�˿��Բ��뱥��̼������Һ��ԭ����
ʵ��� �ⶨ���������Ĵ���
ȡ��ͬѧ��������������Ʒ1.0 g������20.00 mL 0.500 mol��L-1��NaOH��Һ�У��������Сʱ���������η�̪����0.075 mol��L-1���������Һ�ζ����ظ�����ʵ��������������ƽ��ֵΪ20.00 mL��
��4��������������Ʒ�Ĵ���Ϊ
��5���ﵽ�ζ��յ�ʱ����Һ��ɫ�� ��Ϊ
��6�����в���������������������ƫ�͵�ԭ����
| A����ʽ�ζ���δ��ϴ | B����ʽ�ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ |
| C�����������Һʱ�����ݸ��� | D����������δ��ȫˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ�������ĴΣ�12�£��¿���ѧ�Ծ��������棩 ���ͣ������
��12�֣�ij��ȤС��Ϊ�о�������������ȡ����������ʵ�顣
ʵ��һ ���Ҵ���������ȡ��������
�ס���ͬѧ�ֱ�ʹ��ͼ1��ͼ2װ����ȡ����������
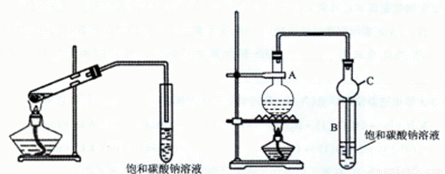
ͼ1 ͼ2
��1����ȡ���������Ļ�ѧ����ʽΪ
��2��������̼������Һ��������������Һ�Ƿ���У�������ԭ��
��3��ͼ1����ĩ�˲��ܲ��뱥��̼������Һ����ͼ2װ���е�����C��ĩ�˿��Բ��뱥��̼������Һ��ԭ����
ʵ��� �ⶨ���������Ĵ���
ȡ��ͬѧ��������������Ʒ1.0 g������20.00 mL 0.500 mol��L-1��NaOH��Һ�У��������Сʱ���������η�̪����0.075 mol��L-1���������Һ�ζ����ظ�����ʵ��������������ƽ��ֵΪ20.00 mL��
��4��������������Ʒ�Ĵ���Ϊ
��5���ﵽ�ζ��յ�ʱ����Һ��ɫ�� ��Ϊ
��6�����в���������������������ƫ�͵�ԭ����
A����ʽ�ζ���δ��ϴ B����ʽ�ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ
C�����������Һʱ�����ݸ��� D����������δ��ȫˮ��
E�����������к������� F����ƿδ��ϴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
( 17 �֣�ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
(l ) A �Թ����������������Ļ�ѧ��Ӧ����ʽΪ��(2��)
_______________________________________________________
(2) ʵ���м����Թܵ�Ŀ���ǣ�(4��)
��
��
(3) ������B�Ĺܿ�û�в��뱥��̼����Һ�����£�ԭ����(2��)________________________________________
(4����ȤС���¼��ʵ������ͽ�����±���
![]()
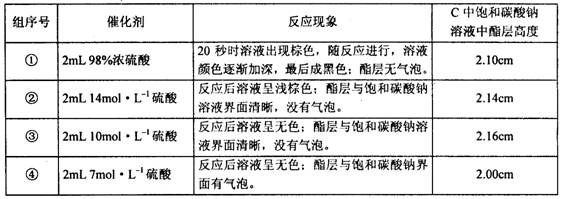
![]()
I���� �� ��ʵ���У��Թ�����Һ��ɫ�淴Ӧ�����������ɺ�ɫ��ԭ���ǣ�
![]()
__________________________________________________________________��(2��)
II���Թ� C ��������û�������ʵ�����ǣ�������ţ�____________________����ʵ����������ѡ�ô��������Ũ����_______________________________��(4��)
����ʵ������֪������_______����ܡ����ܡ�����������Ӧ�Ĵ�������ԭ����____________��(3��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com