
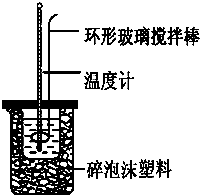
 ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��������ͼװ�ý����к��ȵIJⶨ����ش��������⣺| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
| 1.4212KJ��1mol |
| 0.025mol |


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
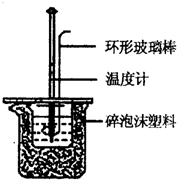 ʵ������50mL 0.50mol/L���ᡢ50mL 0.55mol/L NaOH��Һ����ͼ��ʾװ�ý��вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ������50mL 0.50mol/L���ᡢ50mL 0.55mol/L NaOH��Һ����ͼ��ʾװ�ý��вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ʵ������50mL 0.50mol/L��������50mL 0.55mol/L���ռ���Һ���з�Ӧ��ͨ���ⶨ��Ӧ�����еķ����������к��ȡ��Իش��������⣺
��1���ڱ�ʵ���г����õ����ձ���С�ձ����¶ȼơ���Ͳ�������⣬�����һ�ֲ���������Ϊ ��
��2����ֻ�ձ���Ҫ������ֽ������Ŀ���� ��
��3�����ձ��ϱ������Ӳֽ�壬������õ��к�����ֵ�� ���ƫ����ƫС��������Ӱ�족����
��4��ʵ��ʱ�������ἰNaOH��Һ�������Ϊ50mL������Һ�ܶ�Ϊ1g/cm3��������Һ�ı�����C=4.18J/��g���棩��ʵ����ʼ�¶�Ϊt1�棬��ֹ�¶�Ϊt2�档���ƶ��к��ȵļ���ʽ����H= ��
��5��ʵ���и���52mL0.50mol/L��������50mL0.55mol/L���ռ���Һ��Ӧ��������ʵ����ȣ����ų������� �����ȡ�������ȡ����������к��� �����ȡ�������ȡ�����������
��6������ͬŨ�Ⱥ�����İ�ˮ�����ռ���Һ��������ʵ�飬��õ��к��ȵ���ֵ�� ��������50mL 0.50mol/L�ռ���Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ����ƫС��������Ӱ�족��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10ѧ��֣����ѧ��ѧ�ڸ߶���ĩ���Ի�ѧ�� ���ͣ�ʵ����
��12�֣�ʵ������50mL 0.50mol/L��������50mL 0.55mol/L���ռ���Һ���з�Ӧ��ͨ���ⶨ��Ӧ�����еķ����������к��ȡ��Իش��������⣺
��1���ڱ�ʵ���г����õ����ձ���С�ձ����¶ȼơ���Ͳ�������⣬�����һ�ֲ����� ����Ϊ ��
��2����ֻ�ձ���Ҫ������ֽ������Ŀ���� ��
��3�����ձ��ϱ������Ӳֽ�壬������õ��к�����ֵ�� ���ƫ����ƫС��������Ӱ�족����
��4��ʵ��ʱ�������ἰNaOH��Һ�������Ϊ50mL������Һ�ܶ�Ϊ1g/cm3��������Һ�ı�����C=4.18J/��g���棩��ʵ����ʼ�¶�Ϊt1�棬��ֹ�¶�Ϊt2�档���ƶ��к��ȵļ���ʽ����H= ��
��5��ʵ���и���52mL 0.50mol/L��������50mL 0.55mol/L���ռ���Һ��Ӧ��������ʵ����ȣ����ų������� �����ȡ�������ȡ����������к��� �����ȡ�������ȡ�����������
��6������ͬŨ�Ⱥ�����İ�ˮ�����ռ���Һ��������ʵ�飬��õ��к��ȵ���ֵ�� ��������50mL 0.50mol/L�ռ���Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ����ƫС��������Ӱ�족��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com