
ΓΨΧβΡΩΓΩΚœ≥…Τχ(CO+H2) ΙψΖΚ”Ο”ΎΚœ≥…”–ΜζΈοΘ§ΙΛ“Β…œ≥Θ≤…”ΟΧλ»ΜΤχ”κΥ°’τΤχΖ¥”ΠΒ»ΖΫΖ®ά¥÷Τ»ΓΚœ≥…ΤχΘ§
(1)“―÷Σ±ξΩωœ¬Θ§5.6LCH4”κΥ°’τΤχΆξ»ΪΖ¥”Π ±Έϋ ’51.5kJΒΡ»»ΝΩΘ§«κ–¥≥ωΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ________________ΘΜ
(2)‘Ύ150Γφ ±2L ΒΡΟή±’»ίΤς÷–Θ§ΫΪ2molCH4ΚΆ2mol H2O(g)ΜλΚœΘ§Ψ≠Ιΐ15min¥οΒΫΤΫΚβΘ§¥Υ ±CH4ΒΡΉΣΜ·¬ ΈΣ60%ΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΔΌ¥”Ζ¥”ΠΩΣ Φ÷ΝΤΫΚβΘ§”Ο«βΤχΒΡ±δΜ·ΝΩά¥±μ ΨΗΟΖ¥”ΠΥΌ¬ v(H2)=________ΓΘ
ΔΎ‘ΎΗΟΈ¬Ε»œ¬Θ§ΦΤΥψΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK=________ΓΘ
Δέœ¬Ν–―Γœν÷–Ρή±μ ΨΗΟΖ¥”Π“―¥οΒΫΤΫΚβΉ¥Χ§ΒΡ «________ΓΘ
AΘ°V(H2)Ρφ=3v (CO)’ΐ BΘ°Οή±’»ίΤς÷–ΜλΚœΤχΧεΒΡΟήΕ»≤Μ±δ
CΘ°Οή±’»ίΤς÷–Ήή―Ι«Ω≤Μ±δ DΘ°C(CH4)=C(CO)
(3)Κœ≥…Τχ÷–ΒΡ«βΤχ“≤”Ο”ΎΚœ≥…Α±ΤχΘΚN2+3H2![]() 2NH3ΓΘ±Θ≥÷Έ¬Ε»ΚΆΧεΜΐ≤Μ±δΘ§‘ΎΦΉΓΔ““ΓΔ±ϊ»ΐΗω»ίΤς÷–Ϋ®ΝΔΤΫΚβΒΡœύΙΊ–≈œΔ»γœ¬±μΓΘ‘ρœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «_______________ΘΜ
2NH3ΓΘ±Θ≥÷Έ¬Ε»ΚΆΧεΜΐ≤Μ±δΘ§‘ΎΦΉΓΔ““ΓΔ±ϊ»ΐΗω»ίΤς÷–Ϋ®ΝΔΤΫΚβΒΡœύΙΊ–≈œΔ»γœ¬±μΓΘ‘ρœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «_______________ΘΜ
»ίΤς | ΧεΜΐ | Τπ ΦΈο÷ | ΤΫΚβ ±NH3ΒΡΈο÷ ΒΡΝΩ | ΤΫΚβ ±N2 ΒΡΧεΜΐΖ÷ ΐ | Ζ¥”ΠΩΣ Φ ±ΒΡΥΌ¬ | ΤΫΚβ ±»ίΤςΡΎ―Ι«Ω |
ΦΉ | 1L | 1molN2+3molH2 | 1.6mol | Π’ΦΉ | vΦΉ | PΦΉ |
““ | 1L | 2molN2+6molH2 | n1 mol | Π’““ | v““ | P““ |
±ϊ | 2L | 2molN2+6molH2 | n2 mol | Π’±ϊ | v±ϊ | P±ϊ |
AΘ°n1=n2=3.2 BΘ°Π’ΦΉ=Π’±ϊ>Π’““ CΘ°v““ >v>vΦΉ DΘ°P““>PΦΉ=P±ϊ
(4)Κœ≥…ΤχΩ…“‘÷Τ»ΓΦΉΟ―Θ§¬Χ…ΪΒγ‘¥ΓΑΕΰΦΉΟ―-―θΤχ»ΦΝœΒγ≥ΊΓ±ΙΛΉς‘≠άμ»γœ¬ΆΦΥυ Ψ
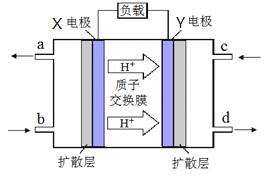
ΔΌ ΒγΦΪY …œΖΔ…ζΒΡΖ¥”Π ΫΈΣ__________ ΘΜ
ΔΎΒγ≥Ί‘ΎΖ≈ΒγΙΐ≥Χ÷–Θ§ΒγΦΪX÷ήΈß»ή“ΚΒΡpH_______(ΧνΓΑ‘ω¥σΓΔΦθ–ΓΓΔ≤Μ±δΓ±)ΓΘ
ΓΨ¥πΑΗΓΩ CH4(g)+H2O(g)=CO(g)+3H2(g) ΓςH= +206kJ/mol 0.12molΓΛL-1ΓΛmin-1 21.87 AC BD O2+4e-+4H+=2H2O Φθ–Γ
ΓΨΫβΈωΓΩ(1). ±ξΩωœ¬5.6LCH4ΈΣ0.25molΘ§‘ρΖΫ≥Χ ΫΈΣ: CH4(g)+H2O(g)=CO(g)+3H2(g)Θ§Έϋ»»Ζ¥”ΠΘ§ΓςHΈΣ’ΐΘ§ΓςH=+51.5ΓΝ4=+206kJ/molΘΜ
(2). ΔΌΦΉΆιΉΣΜ·1.2molΘ§…ζ≥…«βΤχ3.6molΘ§‘ρ«βΤχΒΡ±δΜ·ΝΩΈΣ3.6mol/2L/15min=0.12 molΓΛL-1ΓΛmin-1ΘΜΔΎ![]() ΘΜΔέA. ¥”Ζ¥”ΠΩΣ Φ÷ΝΤΫΚβ«βΤχΒΡ±δΜ·ΝΩΈΣCO(g)ΒΡ3±ΕΘ§A’ΐ»ΖΘΜB. Ζ¥”Π÷ ΝΩ ΊΚψΘ§Έό¬έΖ¥”Π»γΚΈΘ§÷ ΝΩΨυ≤Μ±δΘ§»ίΤςΧεΜΐ≤Μ±δΘ§‘ρΟήΕ»≤Μ±δΘΜC. ΖΫ≥ΧΝΫΕΥΤχΧεΧεΜΐ≤ΜΆ§Θ§»τ»ίΤςΡΎ―Ι«Ω≤Μ±δΘ§‘ρΤχΧεΧεΜΐ≤Μ±δΜ·Θ§Φ¥Ζ¥”Π¥οΒΫΤΫΚβΘ§C’ΐ»ΖΘΜD. Ζ¥”ΠΤΫΚβ ±CH4ΒΡΉΣΜ·¬ ΈΣ≤Μ“ΜΕ®ΈΣ50%Θ§Φ¥C(CH4)= C(CO)≤ΜΡή±Θ÷ΛΖ¥”ΠΤΫΚβΘ§D¥μΈσΓΘΥυ“‘―Γ‘ώACΓΘ
ΘΜΔέA. ¥”Ζ¥”ΠΩΣ Φ÷ΝΤΫΚβ«βΤχΒΡ±δΜ·ΝΩΈΣCO(g)ΒΡ3±ΕΘ§A’ΐ»ΖΘΜB. Ζ¥”Π÷ ΝΩ ΊΚψΘ§Έό¬έΖ¥”Π»γΚΈΘ§÷ ΝΩΨυ≤Μ±δΘ§»ίΤςΧεΜΐ≤Μ±δΘ§‘ρΟήΕ»≤Μ±δΘΜC. ΖΫ≥ΧΝΫΕΥΤχΧεΧεΜΐ≤ΜΆ§Θ§»τ»ίΤςΡΎ―Ι«Ω≤Μ±δΘ§‘ρΤχΧεΧεΜΐ≤Μ±δΜ·Θ§Φ¥Ζ¥”Π¥οΒΫΤΫΚβΘ§C’ΐ»ΖΘΜD. Ζ¥”ΠΤΫΚβ ±CH4ΒΡΉΣΜ·¬ ΈΣ≤Μ“ΜΕ®ΈΣ50%Θ§Φ¥C(CH4)= C(CO)≤ΜΡή±Θ÷ΛΖ¥”ΠΤΫΚβΘ§D¥μΈσΓΘΥυ“‘―Γ‘ώACΓΘ
(3).““»ίΤς÷–ΒΡΤπ Φ―Ι«Ω¥σ”Ύ±ϊ»ίΤς÷–ΒΡΤπ Φ―Ι«ΩΘ§Ι n1n2≤ΜœύΒ»Θ§A¥μΈσΘΜB. »ίΤςΦΉΚΆ±ϊΒΡΤπ Φ―Ι«ΩœύΒ»«“–Γ”Ύ““»ίΤςΘ§Υυ“‘’ΐœρΖ¥”Π≥ΧΕ»Π’““>Π’ΦΉ=Π’±ϊΘ§Υυ“‘N2 ΒΡΧεΜΐΖ÷ ΐΠ’ΦΉ=Π’±ϊ>Π’““Θ§B’ΐ»ΖΘΜC. Ά§BΘ§v““ >v±ϊ=vΦΉΘ§C¥μΈσΘΜD. ”…”ΎΖΫ≥ΧΝΫΕΥΤχΧεΒΡΈο÷ ΒΡΝΩ≤ΜΖΔ…ζ±δΜ·Θ§Υυ“‘ΤΫΚβ ±»ίΤς―Ι«ΩΒ»”ΎΤπ Φ―Ι«ΩΘ§Ι P““>PΦΉ=P±ϊΘ§D’ΐ»ΖΓΘΥυ“‘―Γ‘ώBDΓΘ
(4). ΔΌ“ρΈΣ«βάκΉ”œρYΦΪ“ΤΕ·Θ§‘ρYΦΪΒΟΒΫΒγΉ”ΈΣ’ΐΦΪΘ§”÷»ΦΝœΒγ≥Ί“‘―θΤχΈΣΖ¥”ΠΈοΘ§ΤδΖ¥”ΠΈΣΘΚO2+4e-+4H+=2H2OΘΜΔΎΒγ≥Ί‘ΎΖ≈ΒγΙΐ≥Χ÷–Θ§XΦΪ¥Π…ζ≥…«βάκΉ”Θ§Υυ“‘pHΦθ–ΓΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“‘œ¬ «AΓΪFΈο÷ ΦδΒΡœύΜΞΉΣΜ·ΙΊœΒΆΦΓΘ“―÷ΣAΓΪF÷–ΨυΚ§”–“Μ÷÷œύΆ§ΒΡ‘ΣΥΊ,B «“Μ÷÷ΙΛ“Β≤ζΤΖ,”Π”ΟΙψΖΚ,D‘Ύ≥ΘΈ¬œ¬ «ΤχΧεΓΘC «ΒΞ÷ ,Κή¥ύΒΡΒ≠ΜΤ…ΪΨßΧεΓΘC”κFΈο÷ ΒΡΝΩ÷°±»1ΓΟ1Ζ¥”Π…ζ≥…AΓΘ
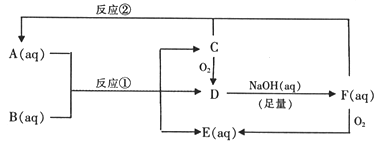
«κΜΊ¥πΘΚ
Θ®1Θ©AΒΡΜ·―ß Ϋ________ΓΘ
Θ®2Θ©Ζ¥”ΠΔΌΒΡάκΉ”ΖΫ≥Χ Ϋ________ΓΘ
Θ®3Θ©ΫΪC”κΙΐΝΩ≈®NaOH»ή“ΚΜλΚœΦ”»»,”–F…ζ≥…ΓΘ–¥≥ωΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–”–ΙΊΈο÷ ”ΟΆΨΒΡΥΒΖ®÷–¥μΈσΒΡ «Θ® Θ©
A.¬»ΤχΩ…”Ο”ΎΉ‘ά¥Υ°ΒΡœϊΕΨ
B.ΥΡ―θΜ·»ΐΧζ≥Θ”ΟΉςΚλ…Ϊ”ΆΤαΚΆΆΩΝœ
C.ΟςΖ·Ω…”Ο”ΎΨΜΥ°
D.Ιΐ―θΜ·ΡΤ”Ο”ΎΚτΈϋΟφΨΏΚΆ«±Υ°Άßάο―θΤχΒΡά¥‘¥
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩC60ΓΔΫπΗ’ ·ΓΔ ·ΡΪΓΔΕΰ―θΜ·ΧΦΚΆ¬»Μ·οΛΒΡΫαΙΙΡΘ–Ά»γΆΦΥυ Ψ( ·ΡΪΫω±μ Ψ≥ωΤδ÷–ΒΡ“Μ≤ψΫαΙΙ):
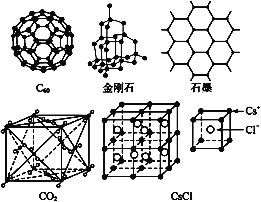
Θ®1Θ©C60ΓΔΫπΗ’ ·ΚΆ ·ΡΪ»ΐ’ΏΒΡΙΊœΒ «ΜΞΈΣ____ΓΘ
A.Ά§Ζ÷“λΙΙΧε B.Ά§ΥΊ“λ–ΈΧε C.Ά§œΒΈο D.Ά§ΈΜΥΊ
Θ®2Θ©ΙΧΧ§ ±,C60 τ”Ύ____(ΧνΓΑ‘≠Ή”Γ±ΜρΓΑΖ÷Ή”Γ±)ΨßΧε,C60Ζ÷Ή”÷–Κ§”–ΥΪΦϋΒΡ ΐΡΩ «____ΓΘ
Θ®3Θ©ΨßΧεΙηΒΡΫαΙΙΗζΫπΗ’ ·œύΥΤ,1 molΨßΧεΙη÷–Κ§”–ΙηΓΣΙηΒΞΦϋΒΡ ΐΡΩ‘Φ «____NAΓΘ
Θ®4Θ© ·ΡΪ≤ψΉ¥ΫαΙΙ÷–,ΤΫΨυΟΩΗω’ΐΝυ±Ώ–Έ’Φ”–ΒΡΧΦ‘≠Ή” ΐ «____ΓΘ
Θ®5Θ©Ιέ≤λCO2Ζ÷Ή”ΨßΧεΫαΙΙΒΡ“Μ≤ΩΖ÷, ‘ΥΒΟςΟΩΗωCO2Ζ÷Ή”÷ήΈß”–____Ηω”κ÷°ΫτΝΎ«“Β»ΨύΒΡCO2Ζ÷Ή”;ΗΟΫαΙΙΒΞ‘ΣΤΫΨυ’Φ”–____ΗωCO2Ζ÷Ή”ΓΘ
Θ®6Θ©Ιέ≤λΆΦ–ΈΆΤ≤β,CsClΨßΧε÷–ΝΫΨύάκΉνΫϋΒΡCs+ΦδΨύάκΈΣa,‘ρΟΩΗωCs+÷ήΈß”κΤδΨύάκΈΣaΒΡCs+ ΐΡΩΈΣ___,ΟΩΗωCs+÷ήΈßΨύάκœύΒ»«“¥ΈΫϋΒΡCs+ ΐΡΩΈΣ___,ΨύάκΈΣ___,ΟΩΗωCs+÷ήΈßΨύάκœύΒ»«“ΒΎ»ΐΫϋΒΡCs+ ΐΡΩΈΣ____,ΨύάκΈΣ____,ΟΩΗωCs+÷ήΈßΫτΝΎ«“Β»ΨύΒΡCl- ΐΡΩΈΣ___ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩPET « άΫγ…œ≤ζΝΩΉν¥σΒΡΚœ≥…œΥΈ§Θ§ΤδΫαΙΙΦρ ΫΈΣΘΚ
![]()
œ÷“‘ΟΚΒΡΗ…Νσ≤ζΤΖA ”κF ΈΣ‘≠Νœ÷Τ±ΗPETΘ§…ζ≤ζΒΡΙΛ“’Νς≥Χ»γΆΦΥυ ΨΓΘ
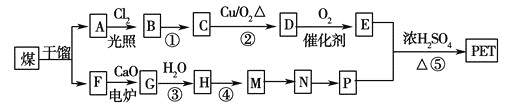
ΔΌAΒΡΖ÷Ή” ΫΈΣC8H10Θ§«“Ρή ΙΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΆ …ΪΘ§ΒΪ≤ΜΡή ΙδεΥ°Ά …ΪΓΘ
ΔΎMΖ÷Ή”άοΥυ”–‘≠Ή”Ι≤ΤΫΟφΓΘ
ΔέG ΈΣCaC2Θ§Τδ”κH2O Ζ¥”Π ±‘ΣΥΊΜ·ΚœΦέ≤Μ±δΓΘ
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)AΒΡΟϊ≥ΤΈΣ__________ΓΘMΓζN ΒΡΖ¥”Πάύ–ΆΈΣ__________ΘΜ
(2)Ζ¥”ΠΔΌΒΡΖ¥”ΠΧθΦΰΈΣΘΚ__________ΘΜ
(3)–¥≥ω”–ΜζΈοA Υυ”–“Μ¬»¥ζΈοΒΡΫαΙΙΦρ ΫΘΚ__________ΓΘ
(4)–¥≥ωœ¬Ν–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌD”κΉψΝΩΒΡ«β―θΜ·Ά≠–ϋΉ«“Κ÷σΖπΘΚ__________ΘΜ
ΔΎΖ¥”ΠΔίΘΚ__________ΓΘ
(5)PΒΡ“Μ÷÷Ά§œΒΈοXΒΡΖ÷Ή” ΫΈΣC3H8O2 Θ§‘ΎΚΥ¥≈Ι≤’ώ«βΤΉΆΦ÷–≥ωœ÷»ΐ÷÷–≈Κ≈ΖεΘ§ΤδΖεΒΡ«ΩΕ»÷°±»ΈΣ2ΓΟ1ΓΟ1ΓΘ‘ρX ΒΡΫαΙΙΦρ ΫΈΣ__________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΒΣΜ·ΟΨ(Mg3N2) ‘ΎΙΛ“Β…œΨΏ”–Ζ«≥ΘΙψΖΚΒΡ”Π”ΟΓΘΡ≥Μ·―ß–Υ»Λ–ΓΉι”ΟΟΨ”κΒΣΤχΖ¥”Π÷Τ±ΗMg3N2≤ΔΫχ––”–ΙΊ Β―ιΓΘ Β―ιΉΑ÷Ο»γœ¬Υυ ΨΘΚ
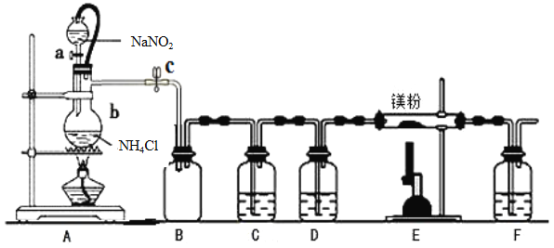
“―÷ΣΘΚΔΌΒΣΜ·ΟΨ≥ΘΈ¬œ¬ΈΣ«≥ΜΤ…ΪΖέΡ©Θ§ΦΪ“Ή”κΥ°Ζ¥”ΠΓΘ
ΔΎ―«œθΥαΡΤΚΆ¬»Μ·οß÷Τ»ΓΒΣΤχΒΡΖ¥”ΠΨγΝ“Ζ≈»»Θ§≤ζ…ζΒΣΤχΒΡΥΌΕ»ΫœΩλΓΘ
ΔέΈ¬Ε»ΫœΗΏ ±Θ§―«œθΥαΡΤΜαΖ÷Ϋβ≤ζ…ζO2Β»ΓΘ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)“«ΤςaΓΔbΒΡΟϊ≥ΤΖ÷±π «____________Θ§____________ΘΜ–¥≥ωΉΑ÷ΟA ÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ____________ΓΘ
(2)ΉΑ÷ΟC÷–ΈΣ±ΞΚΆΝρΥα―«Χζ»ή“ΚΘ§Ής”Ο «_________Θ§ΗΟΉΑ÷Ο÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ____________ΘΜΉΑ÷ΟD ÷–ΒΡ ‘ΦΝ «____________Θ§F ΉΑ÷ΟΒΡΉς”Ο «____________ΓΘ
(3)Φ”»»÷ΝΖ¥”ΠΩΣ ΦΖΔ…ζΘ§–η“ΤΉΏA ¥ΠΨΤΨΪΒΤΘ§‘≠“ρ «____________ΓΘ
(4) Β―ιΫα χΚσΘ§»ΓΉΑ÷ΟEΒΡ”≤÷ ≤ΘΝßΙή÷–ΒΡ…ΌΝΩΙΧΧε”Ύ ‘Ιή÷–Θ§Φ”…ΌΝΩ’τΝσΥ°Θ§Α―»σ ΣΒΡΚλ…Ϊ ·»ο ‘÷ΫΖ≈‘ΎΙήΩΎΘ§Ιέ≤λ Β―ιœ÷œσΘ§ΗΟ≤ΌΉςΒΡΡΩΒΡ «______________ΓΘΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ≤ΌΉςΒΡΡΩΒΡ «__________ΘΜ‘ΌΤζ»Ξ…œ≤ψ«ε“ΚΘ§Φ”»κ―ΈΥαΘ§Ιέ≤λ «Ζώ”–Τχ≈ί≤ζ…ζΘ§ΗΟ≤ΌΉςΒΡΡΩΒΡ «__________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥ –÷–ΩΦΜ·―ß Β―ι≤ΌΉςΩΦ ‘”–ΥΡΗωΩΦΧβΘΚΔΌ’τΝσΘΜΔΎH2ΒΡΜ·―ß–‘÷ ΘΜΔέΕΰ―θΜ·ΧΦΒΡ÷Τ»ΓΓΔ ’Φ·ΚΆ―ι¬ζΘΜΔή―θΤχΒΡ÷Τ»ΓΓΔ ’Φ·ΚΆ―ι¬ζΓΘΩΦ ‘ΒΡΖΫΖ® «”…ΩΦ…ζ≥ι«©»ΖΕ®ΩΦΧβΘ§–ΓΩ≠Ά§―ß≥ι«©Κσ±ΜΦύΩΦάœ Π“ΐΒΦ÷ΝΉΦ±ΗΝΥœ¬Ν–“«ΤςΚΆ“©ΤΖΒΡ Β―ιΧ®«ΑΘΚ

«κΜΊ¥πΘΚ
Θ®1Θ©÷Η≥ω…œΆΦ÷–“«ΤςaΒΡΟϊ≥ΤΘΚ_______ΘΜ
Θ®2Θ©”… Β―ιΧ®…œΧαΙ©ΒΡ“«ΤςΚΆ“©ΤΖΘ§Ρψ»œΈΣ–ΓΩ≠≥ιΒΫΒΡ «ΒΎ____ΗωΩΦΧβΘΜ
Θ®3Θ©“‘œ¬ «–ΓΩ≠Άξ≥…ΗΟ Β―ι÷ς“Σ≤ΌΉςΙΐ≥ΧΒΡ Ψ“βΆΦΓΘΑ¥ΤάΖ÷±ξΉΦΘ§ΟΩœν≤ΌΉς’ΐ»ΖΒΟ1Ζ÷Θ§¬ζΖ÷5Ζ÷Θ§ Β―ιΆξ±œΚσ–ΓΩ≠ΒΟΝΥ3Ζ÷ΓΘ«κ’“≥ωΥϊ ßΖ÷ΒΡ≤ΌΉς≤ΔΥΒΟς‘≠“ρΘΚ______________________ΓΔ______________________ΘΜ
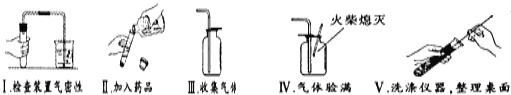
Θ®4Θ©Ϋω”Ο…œ ω“«ΤςΘ®“©ΤΖΝμ―ΓΘ©Θ§“≤ΡήΆξ≥…Νμ“Μ÷÷≥ΘΦϊΤχΧεΒΡ Β―ι “÷Τ»ΓΘ§Μ·―ßΖΫ≥Χ ΫΈΣΘΚ______________ΘΜ»τ‘ωΦ”________ Θ®Χν“Μ÷÷≤ΘΝß“«ΤςΟϊ≥ΤΘ©ΜΙΡήΉιΉΑ≥…ΗΏΟΧΥαΦΊ÷Τ―θΤχΒΡΖΔ…ζΉΑ÷ΟΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΙΛ“Β…œ”ΟΚœ≥…Τχ(COΚΆH2)÷Τ»Γ““¥ΦΒΡΖ¥”ΠΈΣ2CO+4H2![]() CH3CH2OH+H2OΓΘ―–ΨΩΖΔœ÷Θ§ Ι”ΟTiO2ΉςΈΣ‘ΊΧεΗΚ‘ΊννΜυ¥ΏΜ·ΦΝΨΏ”–ΫœΗΏΒΡ““¥Φ≤ζΝΩΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
CH3CH2OH+H2OΓΘ―–ΨΩΖΔœ÷Θ§ Ι”ΟTiO2ΉςΈΣ‘ΊΧεΗΚ‘ΊννΜυ¥ΏΜ·ΦΝΨΏ”–ΫœΗΏΒΡ““¥Φ≤ζΝΩΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©TiΜυΧ§‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ_________ΓΘΚΆOΆ§“Μ÷ήΤΎ«“‘ΣΥΊΒΡΒΎ“ΜΒγάκΡή±»O¥σΒΡ”–______Θ®Χν‘ΣΥΊΖϊΚ≈Θ©Θ§ΚΆOΆ§“Μ÷ήΤΎ«“ΜυΧ§‘≠Ή”ΚΥΆβΈ¥≥…Ε‘ΒγΉ” ΐ±»OΕύΒΡ”–____Θ®Χν‘ΣΥΊΖϊΚ≈Θ©ΓΘ
Θ®2Θ©H2OΖ÷Ή”÷–O‘≠Ή”ΒΡΦέ≤ψΒγΉ”Ε‘ ΐ «________Θ§CH3CH2OHΖ÷Ή”÷–―«ΦΉΜυ(-CH2-)…œΒΡC‘≠Ή”ΒΡ‘”Μ·–Έ ΫΈΣ_______ΓΘ
Θ®3Θ©‘Ύ”ΟΚœ≥…Τχ÷Τ»Γ““¥ΦΖ¥”ΠΥυ…φΦΑΒΡ4÷÷Έο÷ ÷–Θ§Ζ–Βψ¥”ΒΆΒΫΗΏΒΡΥ≥–ρΈΣ_________Θ§‘≠“ρ «__________ΓΘ
Θ®4Θ©ΙΛ“Β…œ“‘COΓΔO2ΓΔNH3ΈΣ‘≠ΝœΘ§Ω…Κœ≥…ΒΣΖ ΡρΥΊ[CO(NH2)2]Θ§CO(NH2)2Ζ÷Ή”÷–Κ§”–ΒΡΠ“Φϋ”κΠ–ΦϋΒΡ ΐΡΩ÷°±»ΈΣ_________ΓΘ
Θ®5Θ©C‘ΣΥΊ”κN‘ΣΥΊ–Έ≥…ΒΡΡ≥÷÷ΨßΧεΒΡΨßΑϊ»γΆΦΥυ ΨΘ®8ΗωΧΦ‘≠Ή”ΈΜ”ΎΝΔΖΫΧεΒΡΕΞΒψΘ§4ΗωΧΦ‘≠Ή”ΈΜ”ΎΝΔΖΫΧεΒΡΟφ–ΡΘ§4ΗωΒΣ‘≠Ή”‘ΎΝΔΖΫΧεΡΎΘ©Θ§ΗΟΨßΧε”≤Ε»≥§ΙΐΫπΗ’ ·Θ§≥…ΈΣ Ή«ϋ“Μ÷ΗΒΡ≥§”≤–¬≤ΡΝœΓΘ
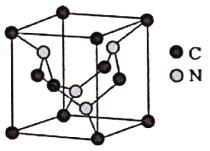
ΔΌΗΟΨßΧε”≤Ε»≥§ΙΐΫπΗ’ ·ΒΡ‘≠“ρ «____________ΓΘ
ΔΎ“―÷ΣΗΟΨßΑϊΒΡΟήΕ»ΈΣd g/cm3Θ§N‘≠Ή”ΒΡΑκΨΕΈΣr1cmΘ§C‘≠Ή”ΒΡΑκΨΕΈΣr2cmΘ§…ηNAΈΣΑΔΖϋΦ”Β¬¬ό≥Θ ΐΘ§‘ρΗΟΨßΑϊΒΡΩ’Φδάϊ”Ο¬ ΈΣ________(”ΟΚ§dΓΔr1ΓΔr2ΓΔNAΒΡ¥ζ ΐ Ϋ±μ ΨΘ©ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΧΦΒΡ―θΜ·ΈοΕ‘ΜΖΨ≥ΒΡ”ΑœλΫœ¥σΘ§CO «»ΦΟΚΙΛ“Β…ζ≤ζ÷–ΒΡ¥σΤχΈέ»ΨΈοΘ§CO2‘ρ¥ΌΫχΝΥΒΊ«ρΒΡΈ¬ “–ß”ΠΓΘΗχΒΊ«ρ…ζΟϋ¥χά¥ΝΥΦΪ¥σΒΡΆΰ–≤ΓΘ
(1)“―÷ΣΘΚΔΌΦΉ¥ΦΒΡ»Φ…’»»ΓςH=-726.4kJΓΛmol-1
ΔΎH2(g)+ ![]() O2(g)=H2O(l) ΓςH=-285.8kJΓΛmol-1ΓΘ
O2(g)=H2O(l) ΓςH=-285.8kJΓΛmol-1ΓΘ
‘ρΕΰ―θΜ·ΧΦΚΆ«βΤχΚœ≥…“ΚΧ§ΦΉ¥ΦΘ§…ζ≥…“ΚΧ§Υ°ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ_____________________________ΓΘ
(2)Εΰ―θΜ·ΧΦΚœ≥…CH3OH ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)ΓςH1Θ§Ιΐ≥Χ÷–Μα≤ζ…ζΗ±Ζ¥”ΠΘΚCO2(g)+H2(g)
CH3OH(g)+H2O(g)ΓςH1Θ§Ιΐ≥Χ÷–Μα≤ζ…ζΗ±Ζ¥”ΠΘΚCO2(g)+H2(g)![]() CO(g)+H2O(g)ΓςH2ΓΘΆΦ1 «Κœ≥…ΦΉ¥ΦΖ¥”Π÷–Έ¬Ε»Ε‘CH3OHΓΔCOΒΡ≤ζ¬ ”Αœλ«ζœΏΆΦΘ§ΓςH2________0(ΧνΓΑΘΨΓ±ΜρΓΑΘΦΓ±)ΓΘ‘ω¥σΖ¥”ΠΧεœΒΒΡ―Ι«ΩΘ§Κœ≥…ΦΉ¥ΦΒΡΖ¥”ΠΥΌ¬ ___________(ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΜρΓΑ ≤Μ±δΓ±)Θ§Η±Ζ¥”ΠΒΡΜ·―ßΤΫΚβ________(ΧνΓΑœρ’ΐΖ¥”ΠΖΫœρΓ±ΓΑœρΡφΖ¥”ΠΖΫœρΓ±ΜρΓΑ≤ΜΓ±)“ΤΕ·ΓΘ
CO(g)+H2O(g)ΓςH2ΓΘΆΦ1 «Κœ≥…ΦΉ¥ΦΖ¥”Π÷–Έ¬Ε»Ε‘CH3OHΓΔCOΒΡ≤ζ¬ ”Αœλ«ζœΏΆΦΘ§ΓςH2________0(ΧνΓΑΘΨΓ±ΜρΓΑΘΦΓ±)ΓΘ‘ω¥σΖ¥”ΠΧεœΒΒΡ―Ι«ΩΘ§Κœ≥…ΦΉ¥ΦΒΡΖ¥”ΠΥΌ¬ ___________(ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΜρΓΑ ≤Μ±δΓ±)Θ§Η±Ζ¥”ΠΒΡΜ·―ßΤΫΚβ________(ΧνΓΑœρ’ΐΖ¥”ΠΖΫœρΓ±ΓΑœρΡφΖ¥”ΠΖΫœρΓ±ΜρΓΑ≤ΜΓ±)“ΤΕ·ΓΘ
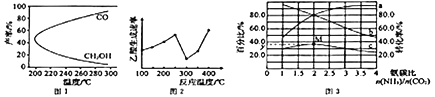
(3)“‘Ρ≥–©ΙΐΕ…Ϋπ τ―θΜ·ΈοΉς¥ΏΜ·ΦΝΘ§Εΰ―θΜ·ΧΦ”κΦΉΆιΩ…ΉΣΜ·ΈΣ““ΥαΘΚCO2(g) +CH4(g)![]() CH3COOH(g) ΓςH=+36.0kJΓΛmol-1ΓΘ≤ΜΆ§Έ¬Ε»œ¬Θ§““ΥαΒΡ…ζ≥…ΥΌ¬ ±δΜ·«ζœΏ»γΆΦ2ΓΘΫαΚœΖ¥”ΠΥΌ¬ Θ§ Ι”Ο¥ΏΜ·ΦΝΒΡΉνΦ―Έ¬Ε» «________ΓφΘ§”ϊΧαΗΏCH4ΒΡΉΣΜ·¬ Θ§«κΧαΙ©“Μ÷÷Ω…––ΒΡ¥κ ©ΘΚ____________________________ΓΘ
CH3COOH(g) ΓςH=+36.0kJΓΛmol-1ΓΘ≤ΜΆ§Έ¬Ε»œ¬Θ§““ΥαΒΡ…ζ≥…ΥΌ¬ ±δΜ·«ζœΏ»γΆΦ2ΓΘΫαΚœΖ¥”ΠΥΌ¬ Θ§ Ι”Ο¥ΏΜ·ΦΝΒΡΉνΦ―Έ¬Ε» «________ΓφΘ§”ϊΧαΗΏCH4ΒΡΉΣΜ·¬ Θ§«κΧαΙ©“Μ÷÷Ω…––ΒΡ¥κ ©ΘΚ____________________________ΓΘ
(4)“ΜΕ®ΧθΦΰœ¬Θ§CO2 ”κNH3 Ω…Κœ≥…ΡρΥΊ[CO(NH2)2]ΘΚCO2(g)+2NH3(g)![]() CO(NH2)2(g)+H2O(g)ΓςHΓΘΡ≥Έ¬Ε»œ¬ΓΘ‘Ύ»ίΜΐΈΣ1LΒΡΚψ»ίΟή±’»ίΤς÷–,Φ”»κ“ΜΕ®Α±ΧΦ±»
CO(NH2)2(g)+H2O(g)ΓςHΓΘΡ≥Έ¬Ε»œ¬ΓΘ‘Ύ»ίΜΐΈΣ1LΒΡΚψ»ίΟή±’»ίΤς÷–,Φ”»κ“ΜΕ®Α±ΧΦ±»![]() ΒΡ3molCO2 ΚΆNH3ΒΡΜλΚœΤχΧεΓΘΆΦ3 «”–ΙΊΝΩΒΡ±δΜ·«ζœΏΘ§Τδ÷–±μ ΨNH3ΉΣΜ·¬ ΒΡ ««ζœΏ________(ΧνΓΑaΓ±ΜρΓΑbΓ±),«ζœΏc ±μ ΨΡρΥΊ‘ΎΤΫΚβΧεœΒ÷–ΒΡΧεΜΐΖ÷ ΐ±δΜ·«ζœΏ,‘ρMΒψΒΡΤΫΚβ≥Θ ΐK=________Θ§y=________ΓΘ
ΒΡ3molCO2 ΚΆNH3ΒΡΜλΚœΤχΧεΓΘΆΦ3 «”–ΙΊΝΩΒΡ±δΜ·«ζœΏΘ§Τδ÷–±μ ΨNH3ΉΣΜ·¬ ΒΡ ««ζœΏ________(ΧνΓΑaΓ±ΜρΓΑbΓ±),«ζœΏc ±μ ΨΡρΥΊ‘ΎΤΫΚβΧεœΒ÷–ΒΡΧεΜΐΖ÷ ΐ±δΜ·«ζœΏ,‘ρMΒψΒΡΤΫΚβ≥Θ ΐK=________Θ§y=________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com