

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A������һ�����ȷŨ�ȵı���Һ |
| B����ȡһ�������Һ�� |
| C����������ƿ������µ����������Һ�� |
| D��ȷϡ��ijһŨ�ȵ���Һ |
 ���ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L��
���ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����25g CuSO4��5H2O����1Lˮ�У������Ƴ�0��1 mol��L-1CuSO4��Һ |
| B������������ȥ�������������л��е������� |
| C���������ſ������ռ�NH��������ʪ�����ɫʯ����ֽ����NH���Ƿ��ռ����� |
| D������Ȳʱ���ñ���ʳ��ˮ����ˮ��Ϊ�˼�����ʯ��ˮ�ķ�Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
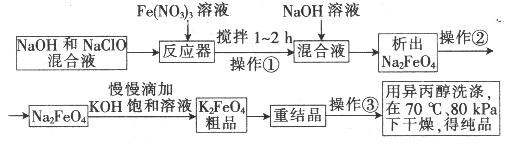
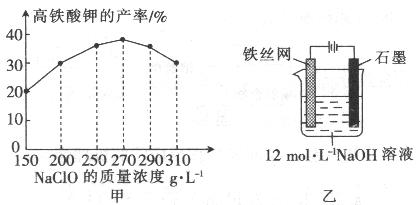
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
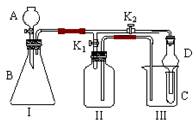
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
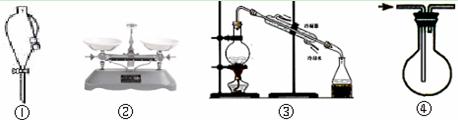
| A��װ�âٿ����ڷ���ú�ͺ�ˮ |
| B��װ�âڿ�����ȷ��ȡ8.55 g�Ȼ��ƹ��� |
| C��װ�âۿ����ڴӺ�ˮ�еõ���ˮ |
| D��װ�âܿ������ſ����ռ�CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Һ�����ʵ���Ũ��Ϊ1 mol��L-1 |
| B������Һ�к���35.5 g Na2SO4 |
| C������100 mL����Һ����7.1 g Na2SO4 |
| D����ȡ100 mL����Һ�����ձ��У��ձ���Na+�����ʵ���Ϊ0.1 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����Cu��ϡ���ᷴӦ��NO |
| B����NaOH������Ũ��ˮ��Ӧ��NH3 |
| C����Fe��ϡ���ᷴӦ��H2 |
| D����MnO2��Ũ���ᷴӦ��Cl2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com