
X��Y��Z��W��Ԫ�����ڱ�ǰ�������е����ֳ���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ���Դ�С��ϵΪ ______________(��д��ѧʽ)�� |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
| Z | Z��Yͬ���ڣ�Z�ĵ縺�Դ���Y |
| W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
(1)Yλ��Ԫ�����ڱ���______���ڵ�______�壬Y��Z������������Ӧ��ˮ��������Խ�ǿ����____________(д��ѧʽ)��
(2)XY2��һ�ֳ��õ��ܼ���XY2�ķ����д���______���Ҽ�����H��Y��H��Z���ֹ��ۼ��У����ļ��Խ�ǿ����________�������ϳ�����_________��
(3)W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��______________________________________
________________________________________________________________________��
(1)3����A��HClO4��(2)2��H��Cl��H��S
(3)[Ar]3d104s1
�������������Ϣ�Ƴ�XԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ��1s22s22p2��Ϊ̼Ԫ�أ�YΪ��Ԫ�أ�ZΪ��Ԫ�أ�WΪͭԪ�ء�
(1)��Ԫ��λ��Ԫ�����ڱ���3���ڵڢ�A�塣(2)XY2ΪCS2���ṹʽΪS===C===S, ����2���Ҽ���H��YΪH��S����H��Z��ΪH��Cl����SԪ�صķǽ���������ClԪ�أ�ԭ�Ӱ뾶S��Cl�����Լ��ļ��Խ�ǿ����H��Cl�������ϳ���ΪH��S��(3)Cuԭ�ӵĺ��������Ϊ29,3d���Ӳ�ȫ����״̬�Ƚ��ȶ����ʻ�̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d104s1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����(����)
A��Na2S2O3��Һ�м���ϡ���S2O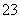 ��2H��===SO2����S����H2O
��2H��===SO2����S����H2O
B����NH4HSO3��Һ�еμ�����KOH��Һ��NH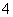 ��OH��===NH3·H2O
��OH��===NH3·H2O
C���μ��(���϶�NaCl��Na2CO3)�м���ʯ�࣬���������ļ��ԣ��漰�ķ���ʽΪCa2����CO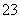 ===CaCO3��
===CaCO3��
D���ں����ҵ���Һ�м��뼸��ϡ���ᣬ�ټ���˫��ˮ����ʹ������Һ������2I����H2O2��2H��===I2��2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ(F)�������Ƹ�Ѫѹ�����ͷʹ��ͻ���Զ�����֢����ϳ�·�����£�
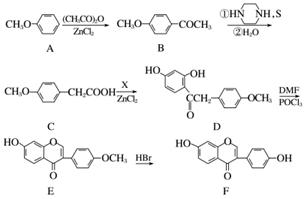
(1)������D�еĺ�����������__________��________��________(�����������)��
(2)��֪��C��DΪȡ����Ӧ������һ����ΪH2O��д��X�Ľṹ��ʽ��________________��
(3)��ӦE��F�ķ�Ӧ������________________��
(4)д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ��________________��
��.���ڷ����廯�����������4�ֲ�ͬ��ѧ��������ԭ��
��.����FeCl3��Һ������ɫ��Ӧ
��.���ܷ���ˮ�ⷴӦ���ܷ���������Ӧ
(5)��������֪ʶ����������Ϣ��д����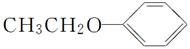 ��(CH3CO)2OΪԭ���Ʊ�ҩ���м���
��(CH3CO)2OΪԭ���Ʊ�ҩ���м��� �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
H2C===CH2HBr,CH3CH2Br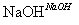 CH3CH2OH
CH3CH2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķ����мȺ��м��Լ����ֺ��зǼ��Լ�����(����)
A��CO2 B��H2O
C��H2O2 D��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���������ָ������Һ�У��ܴ�������Ļ����̡������ܴ�������Ļ�������
(1)���д���Fe3������Һ��Na����SCN����Cl����I��(����)
(2)���д���NO ����Һ��H����Fe2����Cl����SO
����Һ��H����Fe2����Cl����SO (����)
(����)
(3)�����£�pH��12����Һ��K����Cl����SO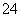 (����)
(����)
(4)c(H��)��0.1 mol·L��1����Һ��Na����NH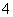 ��SO
��SO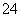 ��S2O
��S2O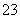 (����)
(����)
(5)ʹpH��ֽ����ɫ����Һ��Cu2����NO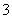 ��Fe3����SO
��Fe3����SO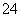 (����)
(����)
(6)�����۷�Ӧ�ų�H2����ɫ��Һ��NO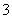 ��Al3����Na����SO
��Al3����Na����SO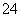 (����)
(����)
(7)ʹ��ɫʯ����ֽ��������Һ��SO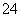 ��CO
��CO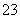 ��Na����K��(����)
��Na����K��(����)
(8)������ ��1��10��12����Һ��K����AlO
��1��10��12����Һ��K����AlO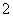 ��CO
��CO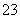 ��Na��(����)
��Na��(����)
(9)������Һ��Fe3����Al3����NO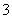 ��SO
��SO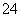 (����)
(����)
(10)ʹ���ȱ��ɫ����Һ��Mg2����K����SO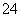 ��SO
��SO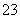 (����)
(����)
(11)c(H��)ˮ��10��12 mol·L��1����Һ��Na����K����CO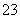 ��SO
��SO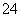 (����)
(����)
(12)ʹ��̪���ɫ����Һ��Na����Cu2����Fe2����NO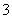 (����)
(����)
(13)0.1 mol·L��1��Na2CO3��Һ��Al3����SO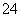 ��Cl����K��(����)
��Cl����K��(����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��M��N������Һ�����ⶨ��������Һ�к�������12�����ӣ�Al3����Cl����Na����K����NO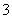 ��OH����Fe2����AlO
��OH����Fe2����AlO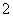 ��CO
��CO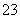 ��NH
��NH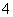 ��SO
��SO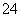 ��H����
��H����
(1)������б�����ʵ��ٵĽ��ۺ�ʵ��ڵ�ʵ�������Լ�����
| ʵ�������Լ����� | ���� |
| ��ȡ����N��Һ�μ����������ᱵ��Һ���������� | |
| �� | ȷ��M��Һ�к���Na��������K�� |
| ����pH��ֽ���M��Һ��pH��ֽ����ɫ |
(2)����(1)�е�ʵ��ش�
NO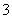 ������________��Һ�У�������________________________________________________________________________��
������________��Һ�У�������________________________________________________________________________��
Cl��������________��Һ�У�������________________________________________________________________________��
(3)����(1)�е�ʵ��ȷ����M��Һ�к��е�����Ϊ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ���ܺ���Na����Ag����Al3����S2����CO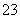 ��SO
��SO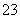 ��NO
��NO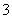 �������е����֡������Һ�м���ϡ���ᣬ��dz��ɫ������������֣�����Һ����ɫΪ��ɫ����������ʵ���������й���ԭ��Һ�����ӳɷֵ��Ʋ���ȷ����(����)
�������е����֡������Һ�м���ϡ���ᣬ��dz��ɫ������������֣�����Һ����ɫΪ��ɫ����������ʵ���������й���ԭ��Һ�����ӳɷֵ��Ʋ���ȷ����(����)
A��һ����S2����SO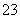 ��Na��
��Na��
B������ֻ��Na����S2����CO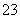
C��һ��û��Ag����Al3��
D��һ����Na����S2����NO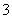
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ�ֵͶ�����Ԫ�أ�������������Ҫ����Ԫ�أ�ʳ�úᵼ�¼����ж�����ʳƷ�����ĺ����������ұ��ͻ���������Σ�������й�����Ԫ�ص�˵����ȷ����(����)
A�����ڿ����в�����������Ϊ�����ʲ�����
B��������������θ�ᷴӦ���������к�θ���ҩ��
C����������������ˮ��ɱ������
D��������麟�����������������ʲ�����ˮ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com