
���� ��1��ʵ����û��480mL������ƿ����Ҫѡ��500mL������ƿ������m=nM�����500mL 0.5mol/L������������Һ�к����������Ƶ�������
��2����������һ�����ʵ���Ũ�ȵ���Һ����ѡ��������Ȼ���жϻ�ȱ�ٵ�������
��3���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=$\frac{n}{v}$�����жϣ�
��� �⣺��1������500mL 0.5mol/L������������Һ����Ҫ�������Ƶ�����Ϊ��m��NaOH��=40g/mol��0.5mol/L��0.5L=10.0g��
�ʴ�Ϊ��10.0��
��2��û�й��Ϊ480mL������ƿ������ʱ��Ҫѡ��500mL������ƿ��ʵ�������Ƶ���500mL 0.5mol/L������������Һ�����Ʋ���Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫʹ�õ������У�������ƽ�����������ձ���ҩ�ס�500mL����ƿ����ͷ�ιܵȣ���ȱ�ٵIJ�������Ϊ��500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��3��A������NaOH�ѳ��⣬ʵ�ʳ������������Ƶ�����ƫС���������Ƶ����ʵ���ƫС������������Һ��Ũ��ƫ�ͣ�
B��С�ձ��ڱڲ�̫�����Ӱ���������Ƶ�����������ҺŨ����Ӱ�죻
C�������Ҫ���ݣ�����ƿϴ����û�к�ɣ�����ҺŨ����Ӱ�죻
D�����������ܽ�ų��������ȣ���Һ�����������������ʣ���Ӧ����ȴ����������Һ���ݣ����Թ���NaOH���ձ����ܽ����������Һת�Ƶ�����ƿ���к���IJ�����ʹ����Һ�������С��������ҺŨ��ƫ�ߣ�
E��ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ��ݣ�������������մ���ձ����벣�����ϣ��������Ƶ�ʵ��������С����ҺŨ��ƫ�ͣ�
F������ʱ����������ƿ�̶��ߣ�ʹ��Һ�����ƫ�ͣ�������ҺŨ��ƫ�ߣ�
G������ҡ�Ⱥ�ֹ��Һ����ڿ̶��ߣ�һ������Һ����ƿ����ƿ��֮�䣬�ټ�����ˮ���̶��ߣ�������Һ���ƫ��������ҺŨ��ƫ�ͣ�
����ƫ�ߵ���DF��ƫ����AEG����Ӱ�����BC��
�ʴ�Ϊ��DF��AEG��BC��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע������ƿ���ѡ��ʹ��ע�������Ŀ�ѶȲ���


 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���Ӿ��� | ԭ�Ӿ��� | ���Ӿ��� | |
| A | NaOH | Ar | SO2 |
| B | H2SO4 | ����� | S |
| C | CH3COONa | ˮ�� |  |
| D | Ba��OH��2 | ���ʯ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
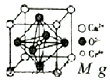 �����仯����㷺Ӧ��������������ƾ������ǵ�ԭ���ǣ�
�����仯����㷺Ӧ��������������ƾ������ǵ�ԭ���ǣ�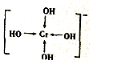 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
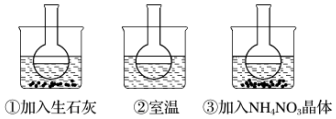
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 12753I��13153I����������ͬ��ͬ�ֺ��� | |
| B�� | �ṹ��������Ƶ����ʣ��е�����Է���������������ߣ�����NH3�е����PH3 | |
| C�� | ʵ������ȡ������Ϊ�˼ӿ췴Ӧ���ʣ�����ϡH2SO4�еμ�����Cu��NO3��2��Һ | |
| D�� | Ϊ������¯ˮ���е�CaSO4�������ñ���Na2CO3��Һ���ݣ��ټ��������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ˮ��Fe3+��NH4+��SO42-��OH- | |
| B�� | �������NaClO��Һ��Fe2+��H+��Cu2+��SO42- | |
| C�� | �������NaOH��Һ��Na+��AlO2-��SO42-��OH- | |
| D�� | �������NaHCO3��Һ��Na+��Al3+��SO42-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 CO2��������ɺϳ���ϩ��2CO2 ��g��+6H2��g��?C2H4��g��+4H2O��g����0.1MPaʱ����n��CO2����n��H2��=l��3Ͷ�ϣ���ò�ͬ�¶���ƽ��ʱ��ϵ�и�����Ũ�ȵĹ�ϵ��ͼ��������������ȷ���ǣ�������
CO2��������ɺϳ���ϩ��2CO2 ��g��+6H2��g��?C2H4��g��+4H2O��g����0.1MPaʱ����n��CO2����n��H2��=l��3Ͷ�ϣ���ò�ͬ�¶���ƽ��ʱ��ϵ�и�����Ũ�ȵĹ�ϵ��ͼ��������������ȷ���ǣ�������| A�� | �÷�Ӧ�ġ�H��O | |
| B�� | ����b����H2O | |
| C�� | N���M������״̬��c��H2����һ�� | |
| D�� | �����������䣬T1�桢0.2 MPa�·�Ӧ��ƽ��ʱc��H2����M��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com