

 ��
��
���� 2-��ϩAΪCH3CH=CHCH3��������Ϣ�٢ۿ�֪��A��B������˫ȡ������Ӧ��Ϊ��CH3CH=CHCH3+2Cl2$\stackrel{500��}{��}$CH2ClCH=CHCH2Cl+2HCl��
±�����ڼ���������ˮ�⣺B��C����Ӧ��Ϊ��CH2ClCH=CHCH2Cl+2NaOH$\stackrel{��}{��}$CH2OHCH=CHCH2OH+NaCl��C��CH2OHCH=CHCH2OH������Է�������Ϊ88��
������Ϣ�ڿ�֪����ʹC��ijЩ���ʷ����ӳɣ�����̼̼˫��������C��D����Ӧ��Ϊ��CH2OHCH=CHCH2OH+HBr��CH2OHCH2CHBrCH2OH��
��Ӧ��Ϊ���ǻ���������D��E��CH2OHCH2CHBrCH2OH+O2$��_{��}^{����}$CHOCH2CHBrCHO+2H2O��
��Ӧ��Ϊȩ����������E��F��CHOCH2CHBrCHO+O2$��_{��}^{����}$COOHCH2CHBrCOOH��
��Ӧ��Ϊ±��ԭ�����������ƵĴ���Һ����ȥ��F��G��COOHCH2CHBrCOOH+NaOH$��_{H+}^{��/��}$ COOHCH=CHCOOH+NaBr��G��COOHCH=CHCOOH������Է�������Ϊ116����G������ֻ��2����ԭ�ӣ�
��Ӧ��Ϊϩ���ļӾ۷�Ӧ��G��H��nCOOHCH=CHCOOH$\stackrel{һ������}{��}$ ���ݴ˷������
���ݴ˷������
��� �⣺��1��ȡ����Ӧָ���л�������������ijЩԭ�ӻ�ԭ���ű�����ԭ�ӻ�ԭ����������ķ�Ӧ������Ӧ��Ϊ��CH3CH=CHCH3˫�����ڵ�̼ԭ���ϵ�һ����ԭ�ӱ���ԭ��ȡ����
��Ӧ��Ϊ��CH2ClCH=CHCH2Cl��ԭ�ӱ��ǻ�ȡ������ȥ��Ӧָ���л�������ȥһ����С���ӣ���ˮ��±����ȷ��ӣ��������ɲ����ͣ�̼̼˫����������״��������ķ�Ӧ����Ӧ��ΪCOOHCH2CHBrCOOH±��ԭ����ԭ�����������ƵĴ���Һ�з�����ȥ��
�ʴ�Ϊ���٢ڣ��ޣ�
��2����Ӧ�۱���̼̼˫������ʹC��ijЩ���ʷ����ӳɣ���Ӧ��Ϊ��CH2OHCH=CHCH2OH+HBr��CH2OHCH2CHBrCH2OH�����ӵ��Լ�Ϊ�廯�⣬
�ʴ�Ϊ��HBr��
��3����Ӧ��Ϊ���ǻ���������D��E��CH2OHCH2CHBrCH2OH+O2$��_{��}^{����}$CHOCH2CHBrCHO+2H2O��EΪCHOCH2CHBrCHO�����еĹ�����Ϊ��ԭ�ӣ�-Br����ȩ����-CHO����
�ʴ�Ϊ����ԭ�ӣ�-Br����ȩ����-CHO����
��4����Ӧ��Ϊϩ���ļӾ۷�Ӧ��G��H��nCOOHCH=CHCOOH$\stackrel{һ������}{��}$ ������HΪ��
������H�� ��
��
�ʴ�Ϊ�� ��
��
��5���Ա�ϩ��CH3CH=CH2��Ϊ��Ҫԭ�Ͽ��Ժϳɸ��ͣ�����Ϊ��������������Ϣ�ٿ�֪����˫�����ڵ�̼ԭ��������±��ԭ�ӣ�Ȼ��˫����±��ԭ�Ӽӳɣ����±��ԭ�����������Ƶ�ˮ��Һ��ˮ�⼴�ɵõ����ͣ����Է�Ӧ����Ϊ��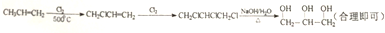 ��
��
�ʴ�Ϊ��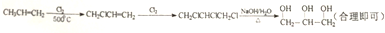 ��
��
���� ���⿼���л���ĺϳ����ƶϣ����������Ϣ��ԭ����Ҫȡ����˫�����ڵ�̼ԭ���ϵ�һ����ԭ��Ϊ������Ĺؼ�֮����ע����ճ����л���Ĺ����ź����ʣ���Ŀ�Ѷ��еȣ�


 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ����� | �Լ� | ���뷽�� | |
| �� | �������ӣ� | ����������Һ | ��Һ |
| �� | ���飨��ϩ�� | ���Ը��������Һ | ϴ�� |
| �� | �������������ᣩ | ����������Һ | ���� |
| �� | ��Ȳ�����⣩ | ����ͭ | ϴ�� |
| A�� | �ڢۢ� | B�� | �ڢ� | C�� | �ۢ� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Ϊ̽����������NaOH�Ҵ���Һ������Ӧ�����ɵ��������Ƿ���������װ����ͼ��ʾ���ش�
Ϊ̽����������NaOH�Ҵ���Һ������Ӧ�����ɵ��������Ƿ���������װ����ͼ��ʾ���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ӵ���������6.02��1023 | |
| B�� | ���ʵ�����һ����������ʾ����һ����Ŀ���ӵļ��� | |
| C�� | Ħ�����߸�����������֮һ | |
| D�� | Ħ���������ʵ����ĵ�λ���������ӵ�������λ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com