
��14�֣����������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��
��֪����4FeO?Cr2O3+8Na2CO3+7O2 8Na2CrO4+2Fe2O3+8CO2����
8Na2CrO4+2Fe2O3+8CO2����
��Na2CO3+Al2O3 2NaAlO2+CO2����
2NaAlO2+CO2����
��Cr2CO72-+H2O 2CrO42-+2H+
2CrO42-+2H+
��������ش��������⣺
��1������X����Ҫ���� ����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4��5��Ӧ��ʹ�� ______________ ����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH��5����Ŀ���� _______________________ ��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� �����ˡ� �����
��4���±���������ʵ��ܽ�����ݣ�����III������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7 + 2KCl��K2Cr2O7 ��+ 2NaCl���÷�Ӧ����Һ���ܷ�����������_____________________________��
| ���� | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 | |
| �ܽ�ȣ�g/100gˮ�� | 0�� | 28 | 35��7 | 4��7 | 163 |
| 40�� | 40��1 | 36��4 | 26��3 | 215 | |
| 80�� | 51��3 | 38 | 73 | 376 | |
(13��)��1��Fe2O3��MgO(2��)PH�ƻ�pH��ֽ(1��)��2��ʹCrO42-ת��ΪCr2O72-(1��)
��3����ȴ�ᾧ��ϴ��(��1��)
��4��K2Cr2O7���ܽ�ȱ�Na2Cr2O7С��������������K2Cr2O7���ܽ����С��(2��)
��5��NaOH��Һ��ͨ�����������̼��(��1�֣�������Ҳ����)��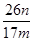 (3��)
(3��)
���������������1�����������Ҫ�ɷֿɱ�ʾΪFeO?Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ�����������̼���ƣ�������ӦΪ����4FeO?Cr2O3+8Na2CO3+7O2 8Na2CrO4+2Fe2O3+8CO2������Na2CO3��Al2O3
8Na2CrO4+2Fe2O3+8CO2������Na2CO3��Al2O3 2NaAlO2��CO2������Cr2CO72-+H2O
2NaAlO2��CO2������Cr2CO72-+H2O 2CrO42-+2H+���������ǹ��˵õ�����XΪFe2O3��MgO��Ҫ����ữ��������Һ��pH�Ƿ����4��5����ͨpH��ֻֽ�ܲⶨ��ҺpH���������ǽ��Ʋⶨ��ȷ�ⶨ��Ҫ��pH�ƻ�ȷpH��ֽ��
2CrO42-+2H+���������ǹ��˵õ�����XΪFe2O3��MgO��Ҫ����ữ��������Һ��pH�Ƿ����4��5����ͨpH��ֻֽ�ܲⶨ��ҺpH���������ǽ��Ʋⶨ��ȷ�ⶨ��Ҫ��pH�ƻ�ȷpH��ֽ��
��2���ữ�����ô��������ҺpH��5����������ͼ�����ʵ�ת�����Ʊ�Ŀ�Ŀ�֪����Ϸ�Ӧƽ��Cr2O72-+H2O 2CrO42-+2H+�������ᣬ������Ũ������ƽ�����ƣ�������ʹCrO42-ת��ΪCr2O72--��
2CrO42-+2H+�������ᣬ������Ũ������ƽ�����ƣ�������ʹCrO42-ת��ΪCr2O72--��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ����壻
��4������ͼ�����ʵ��ܽ�ȷ����Աȣ�����������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl��K2Cr2O7��+2NaCl����˵��K2Cr2O7���ܽ�ȱ�Na2Cr2O7С��������������K2Cr2O7���ܽ����С����
��5������ƷY��Ҫ��������������������þ���������ܻ����P���������ʣ���ȷ����Y���������������ķ������������������������ԣ�������������Һ�ܽ������������˵õ���Һ��ͨ�������̼���������������������ո���õ��������������m g��������Ԫ���غ���㣬��Ʒ����������������������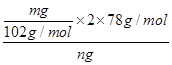 ��100%��
��100%��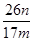 ��
��
���㣺���������Ʊ����̺ͷ����ķ����жϣ��������ʵ�Ӧ�õ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������������©��������ƿ��������ƿ����ƽ�ݷ�Һ©���ζ��ܢ�ȼ�ճף����������ʷ������(����)
| A���٢ۢ� | B���٢ڢ� | C���٢ۢ� | D���ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ȣ�Sr��Ϊ�������ڢ�A��Ԫ�أ��仯������ˮ�Ȼ��ȣ�SrCl2��6H2O����ʵ������Ҫ�ķ����Լ�����ҵ�ϳ�������ʯ����Ҫ�ɷ�ΪSrSO4��Ϊԭ���Ʊ��������������£�
��֪���� �������ȡ����Һ�г�����Sr2+��Cl���⣬����������Ba2+���ʣ�
�� SrSO4��BaSO4���ܶȻ������ֱ�Ϊ3.3��10��7��1.1��10��10��
�� SrCl2��6H2O��Ħ������Ϊ��267 g/mol��
��1������ʯ����ǰ����ĥ���飬��Ŀ����_________________________________________��
��2�������������±��գ���0.5 mol SrSO4��ֻ��S����ԭ��ת����4 mol���ӡ�д���÷�Ӧ�Ļ�ѧ����ʽ��_____________________________________________��
��3��Ϊ�˵õ��ϴ�����ˮ�Ȼ��Ⱦ��壬����2������е�����������_______________��
��4�����������Ŀ����______________________��Ϊ�����ԭ�ϵ������ʣ���Һ��Sr2+��Ũ��Ӧ������_________ mol/L��ע����ʱ��Һ��Ba2+Ũ��Ϊ1��10��5 mol/L����
��5����Ʒ���ȼ�⣺��ȡ1.000 g��Ʒ�ܽ�������ˮ�У������м��뺬AgNO3 1.100��10��2 mol��AgNO3��Һ����Һ�г�Cl�D�⣬����������Ag+��Ӧ�����ӣ�����Cl�D��ȫ�������ú�Fe3+����Һ��ָʾ������0.2000 mol/L��NH4SCN����Һ�ζ�ʣ���AgNO3��ʹʣ���Ag+ ��AgSCN��ɫ��������ʽ������
�ٵζ���Ӧ�ﵽ�յ��������_________________________________________��
�����ζ�������ȥ����Ũ�ȵ�NH4SCN��Һ20.00 mL�����Ʒ��SrCl2��6H2O�������ٷֺ���Ϊ______________������4λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�˻������ø������������в�����SO2����ҵ�ϲ������̿���Ҫ�ɷ�MnO2������ͬʱ��ȡ�����̼�����������ʾ��ͼ���£�
��֪������Һ��pH�ӽ�4�����еĽ���������Ҫ��Mn2����������������Fe2�������������ա�
��1��д��������������Ҫ��Ӧ�Ļ�ѧ����ʽ�� ��
��2���������̵ĸ���Ӧ֮һ�Dz���SO2������Ϊ���ᣬ��ʹ����Һ��pH�½����⽫ ���������������������������̿�������SO2�����������ɵ����ᣬ�Լ�A����� ��
a��MnCO3 b��MnO2 c��CaO d��CaCO3
��3������I��Ŀ���dz�ȥ����Һ�е�Fe2����MnO2������Fe2����ͬʱ�� ��ʹFe3��������������˺���Һ���Ƿ���Fe3+�IJ����� ��
��4����֪����27��ʱ��MnSO4��H2O�ܽ�����¶������������½��������II�Ĺ���Ϊ�� �� ��ϴ�ӡ������ҵ��Ϊ�˳��������Ԫ�أ��������� ��ѭ��ʹ�á�
��5��ͨ������MnSO4��H2O ���Ƶ�����������������ϵ�MnxO4����ͼ������MnSO4��H2Oʱ�¶���ʣ����������仯���ߡ���������A������ʾ���ʵĻ�ѧʽΪ ��MnxO4��x�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺,���������ʵ��ش�����:
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и�������,�����д��ԵĹ���Y,��Y���ڹ����������Һ�к��еĴ�������������������(2)ij��Һ����Mg2+��Fe2+��Al3+��Cu2+����������,�����м��������NaOH��Һ��,����,�������������ղ������պ�Ĺ���Ͷ�������ϡ������,������Һ��ԭ��Һ���,��Һ�д������ٵ�����������������A.Mg2+ B.Fe2+
C.Al3+ D.Cu2+
(3)����������Ҫ��ҵ����,�÷���м�Ʊ�������������:
�ش���������:
�ٲ������������������,�����������������������д���ڿ���������FeCO3�Ļ�ѧ����ʽ������������������������������(4)��Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��
a.��ȡ2.850 g�̷�(FeSO4��7H2O)��Ʒ,�ܽ�,��250 mL����ƿ�ж���;
b.��ȡ25.00 mL������Һ������ƿ��;
c.�������ữ��0.010 00 mol��L-1KMnO4��Һ�ζ����յ�,����KMnO4��Һ�����ƽ��ֵΪ20.00 mL��
��ʵ��ǰ,����Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL,����ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι���,��������������ijͬѧ��Ƶ����еζ���ʽ,�����������������(�гֲ�����ȥ)(����ĸ���)
��д���ζ������з�Ӧ�����ӷ���ʽ�����������������ܼ���������Ʒ��FeSO4��7H2O����������Ϊ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ�ӵ�кܳ��ĺ����ߣ����зḻ�ĺ�����Դ���Ժ�ˮΪԭ�ϵ��λ������ҹ���Ҫ�IJ�ҵ����±�Ǻ�ˮɹ�κ�ĸ���þ�ε���Һ�����г���þ���⣬��������������(����ͼ��)����±��ʳƷ�������ȷ�����й㷺����;��������������±�йص����⡣
(1)����ͼ�ף�д����±�к������������εĻ�ѧʽ�� �� ��
(2)��ͼ������±��ijЩ���ʵ��ܽ�����ߣ���֪T1��ʱ��MgSO4��KCl���ܽ�ȷֱ�ΪM��N���������ܽ�ȴ�С�Ĺ�ϵΪ ������±���ȵ�T2�����ϣ������ܽ�����ߣ����ȴ���±�з�������ľ����� ��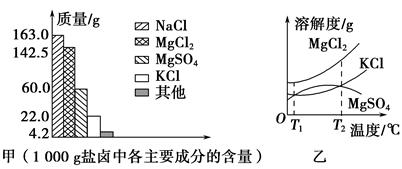
(3)����������±ˮ��������þ�Ĺ����������£�
�������١���Ŀ���ǽ�������þ������������������ٵ������� ��������þ���Լ�B��Ӧ�Ļ�ѧ����ʽΪ ��
�����ڵ������� ���÷������ŵ�Ϊ ��
(4)�õ�ⷨ�Ʊ�����þ����λͬѧ�ֱ��������������ַ�����
�ף�����Ȼ�þ��Һ��
�ң�������ڵ��Ȼ�þ��
����ͬѧ (��ס����ҡ�)�ܹ��ɹ����Ʊ�����þ����������������þԪ��û����ʧ����100 g±ˮ���Ʊ�þ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ͳ�������Fe�۾��г�ǿ�Ĵ��ԣ����������ܶȴż�¼�Ľ����Լ���Ч �����ȡ�
I��ʵ���Ҳ������ԭ���Ʊ�����Fe,���������£�
��1�� ���������ˮͨ��Ҫͨ�� ��
���������ˮͨ��Ҫͨ�� ��
��2����������Fe�Ļ�ѧ����ʽΪ ��
���ڲ�ͬ�¶��£�����Fe����ˮ������Ӧ�Ĺ�����ﲻͬ���¶ȵ���570��ʱ����FeO������570��ʱ���� ����ͬѧ����ͼ��ʾװ�ý�������Fe����ˮ�����ķ�Ӧ����֤������
����ͬѧ����ͼ��ʾװ�ý�������Fe����ˮ�����ķ�Ӧ����֤������
��3��B���ռ����������� (������)��Cװ�õ������� ��
��4����ͬѧΪ̽��ʵ��������Թ��ڵĹ������ʳɷ֣�����������ʵ�飺
��ͬѧ��Ϊ�������·�Ӧ�Ĺ������ΪFeO����ͬѧ��Ϊ�ý��۲���ȷ������������ (�����ӷ���ʽ��ʾ)��
��5����ͬѧ��ȡ5��60gFe�ۣ���Ӧһ��ʱ���ֹͣ���ȡ����Թ��ڵĹ��������ڸ���������ȴ�Ƶ�����Ϊ6��88g��Ȼ����ȴ��Ĺ�������������FeCl3��Һ��ַ�Ӧ������0��08molFeCl3����ͬѧʵ��Ĺ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
±�����Ҫ�ɷ���MgCl2������� Fe3+��Fe2+��Mn2+�����ӡ���±��Ϊԭ�Ͽ��Ƶ���������þ��������������ͼ��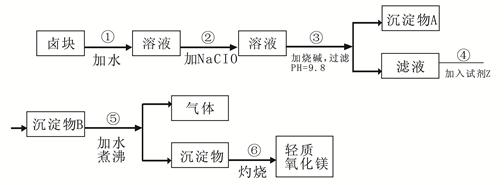
��֪��Fe2+�����������״�����״���Һ�г�ȥ�����Գ���������ΪFe3+������Fe(OH)3������ȥ����Ҫ���Ʒ�����������ʣ�����ݱ�1��2�ṩ�����ϣ���д�հף�
��1 �����������������pH
| ���� | ��ʼ���� | ������ȫ |
| Fe��OH��3 | 2.7 | 3.7 |
| Fe��OH��2 | 7.6 | 9.6 |
| Mn��OH��2 | 8.3 | 9.8 |
| Mg��OH��2 | 9.6 | 11.1 |
| �Լ� | �۸�Ԫ/�֣� |
| ƯҺ����NaClO��25.2%�� | 450 |
| ˫��ˮ����H2O2 ,30%�� | 2400 |
| �ռ��98% NaOH�� | 2100 |
| �����99.5% Na2CO3�� | 600 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ���ḻ����Դ���⣬ͨ����ˮ���ۺ����ÿɻ���������ʹ�����ʹ�á�
��1�� ��ˮ���εĿ������ã�
��.��ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���뺣�ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ�_______�ء�
II.Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ������____________________________________________����дһ�㼴�ɣ�
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ������ͼ��ʾ����ش��������⣺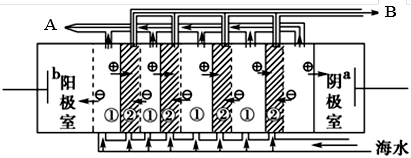
��.��ˮ����ֱ��ͨ�뵽��װ���У�������_____________________________________________��
��. B���ų�����________(���ˮ����Ũˮ��)��
��3���ÿ�±����Na+��K+��Mg2+��Cl-��Br-�����ӣ�����ȡ�壬�������������£�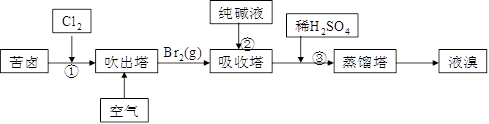
��.���������е���Һ��BrO3�������������з�Ӧ�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣ�
_________________________________________��
��.ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��_____________________________________________________________________��
��.����������ͨ��ˮ�������ȣ������¶���900C���ҽ��������ԭ����___________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com