
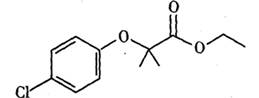
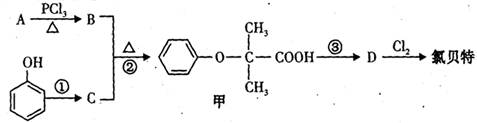
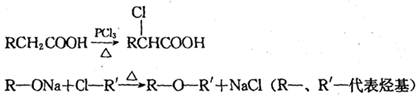
 d.
d.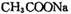
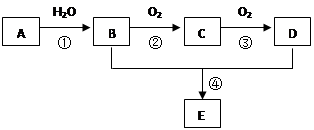
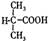 ���Ȼ�����ԭ��(3) c��d (4)���֡�
���Ȼ�����ԭ��(3) c��d (4)���֡�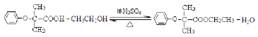 ��D
��D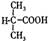 ��BΪ
��BΪ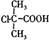 ��B�к��еĹ���������Ϊ�Ȼ�����ԭ�ӡ�(3)���ӵ����Խ�����ֻ����NaOH��Na2CO3 ��Ӧ��������cd�������ʲ��ܴﵽĿ�ġ�(4)�Ӻϳ�·�߿�֪����Ӧ�ڵķ�Ӧ����ӦΪȡ����Ӧ���ɡ����������Ҽ�����FeCl3��Һ����ɫ�����ܷ���������Ӧ��˵��ͬ���칹�庬��������ȩ���ͷ��ǻ����ɡ�1molX�������3molNaOH��Ӧ��˵��ͬ���칹���ˮ��������������3molNaOH����ͬ�ֹ�����
��B�к��еĹ���������Ϊ�Ȼ�����ԭ�ӡ�(3)���ӵ����Խ�����ֻ����NaOH��Na2CO3 ��Ӧ��������cd�������ʲ��ܴﵽĿ�ġ�(4)�Ӻϳ�·�߿�֪����Ӧ�ڵķ�Ӧ����ӦΪȡ����Ӧ���ɡ����������Ҽ�����FeCl3��Һ����ɫ�����ܷ���������Ӧ��˵��ͬ���칹�庬��������ȩ���ͷ��ǻ����ɡ�1molX�������3molNaOH��Ӧ��˵��ͬ���칹���ˮ��������������3molNaOH����ͬ�ֹ�����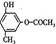 ��
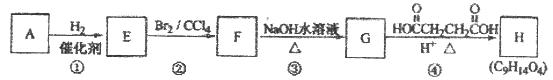
��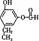 ���֡�(5)�Ӻϳ�·�߿�֪��D�Ǽ����Ҵ�����������Ӧ�IJ������ʽΪ
���֡�(5)�Ӻϳ�·�߿�֪��D�Ǽ����Ҵ�����������Ӧ�IJ������ʽΪ ��
��

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
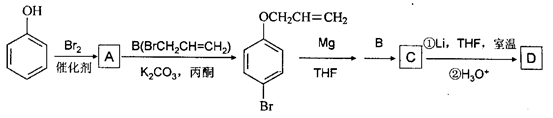
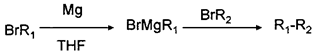 ��THFΪһ���л��ܼ���
��THFΪһ���л��ܼ���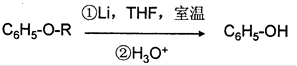
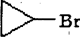 �ǻ�����B��һ��ͬ���칹�壬��1H�˴Ź�������֤���û���������______����ڲ�ͬ�Ļ�ѧ������
�ǻ�����B��һ��ͬ���칹�壬��1H�˴Ź�������֤���û���������______����ڲ�ͬ�Ļ�ѧ������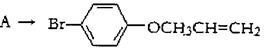 �л���Ӧ���ͣ�___________��
�л���Ӧ���ͣ�___________��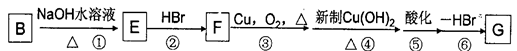
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
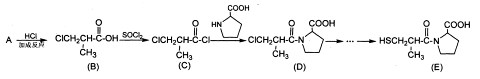
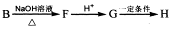 ���߷��ӻ��������
���߷��ӻ��������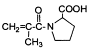 �ж���ͬ���칹�壬��
�ж���ͬ���칹�壬��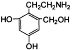 �ȡ�д��������������������ͬ���칹�壺
�ȡ�д��������������������ͬ���칹�壺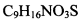
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
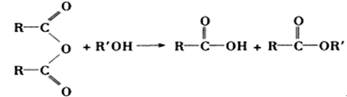
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
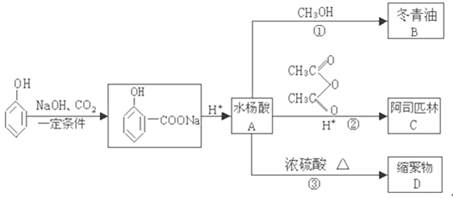
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
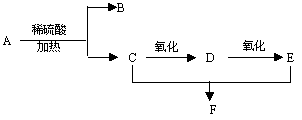
 ��CH3CH2CHO + Cu(OH)2
��CH3CH2CHO + Cu(OH)2 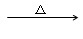
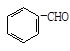
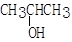
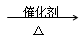
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��Ϊ
��Ϊ
|
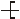
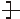 n�����䵥��ΪA����ϩ
n�����䵥��ΪA����ϩ B��1��3-����ϩ C����Ȳ D������
B��1��3-����ϩ C����Ȳ D�������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com