
ijУ����С��Ϊ̽��ͭ��ϡ���ᷴӦ������������Ҫ��NO�����������ʵ�顣ͼ��KΪֹˮ�У�F��װ��һ�������ע���������м���װ�ú̶�װ�þ�����ȥ��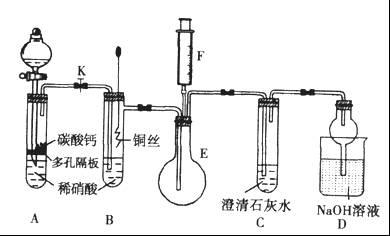
��ش��й����⣺
��1�����װ��A��Ŀ����_______________________��
��2�������װ��A��ʵ��Ŀ�ĺر�K���ٽ�װ��B�е�ͭ˿����ϡ���ᡣB�з�Ӧ�����ӷ���ʽ��____________________________��
��3����F�еĿ�������E�У�֤��NO���ڵ�ʵ��������_______________���˹��̷�����Ӧ�Ļ�ѧ����ʽ��____________________��
��4��װ��D��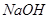 ��Һ��������________________��
��Һ��������________________��
��1���������ɵĶ�����̼�Ͼ�����װ���ڵĿ����������NO�ļ�����ɸ��š���2�֣�
��2��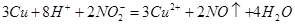 ��2�֣�
��2�֣�
��3��E����ɫ�����Ϊ����ɫ 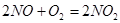 ��2�֣�
��2�֣�
��4�����ն���ĵ��������ֹ��Ⱦ������2�֣�
�������������A ��ȡ������̼���߿�������ֹ����һ�������ļ��顣B ����ȡһ��������װ�á�E һ������ת������������װ�á�C ��������̼��װ�á�D �����յ����������װ�á�
���㣺������һ����������ȡ��ת����ʵ�飬�����˵�������������ʡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�ʵ��װ�á�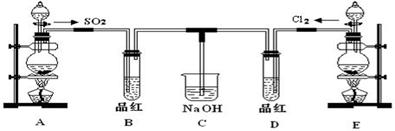
��1��ʵ������װ��A�Ʊ�SO2��ijͬѧ��ʵ��ʱ���ִ�A�ķ�Һ©��������©����Һ��δ���£�����Ϊԭ������� ��
��2��ʵ������װ��E�Ʊ�Cl2���䷴Ӧ�Ļ�ѧ��ѧ����ʽΪ��
MnO2+4HCl��Ũ�� MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
��3���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�B�� ��D�� ��
��ֹͣͨ����,�ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�ΪB�� ��D�� ��
��4����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��Cl2��1��1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ���������������������������������ԭ���û�ѧ����ʽ��ʾ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������Ϸ��֣�N2��ȡ�����в�ͬ������
a����������������NH3��ԭCuO�Ƶô�����N2�ͻ���ͭ��
�ⷽ��������NaNO2��NH4Cl��Ũ��Һ�Ƶ�
c������������������ͨ�����ȵ�ͭ���Ƶýϴ���N2
����ʵ�����й�ѡ������¼�����������ȡN2
��1������ ������N2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲������������е�____________����A��B��C������ͬ����NH3�ķ�������Ҫ��ȡ���ռ�N2������ѡ�õ���������___________��
������N2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲������������е�____________����A��B��C������ͬ����NH3�ķ�������Ҫ��ȡ���ռ�N2������ѡ�õ���������___________��
��2��д��b�����з�Ӧ�Ļ�ѧ����ʽ_________________________
��3������ ������ȡN2��������һ�����к����������� ______________________
������ȡN2��������һ�����к����������� ______________________
��4��������N2�����������У�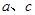 �������ʹ��Խ��Խ�ܵ����ǵĹ�ע�����ַ�����
�������ʹ��Խ��Խ�ܵ����ǵĹ�ע�����ַ����� ������ȣ�����Խ������____________��
������ȣ�����Խ������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����������������ѧ��ѧ�еij������壬̽�����ߵ��Ʒ���������ʮ����Ҫ�Ŀ��⡣
��1��ʵ���ҿ��ɶ���;����ȡSO2��
;��I��Na2SO3�������Ũ�����ᣨԼ70%����Ӧ��ȡ��
;��II��ͭ��Ũ���������ȡ��
���Ҫ��ʵ������ȡSO2��ѡ���������ַ����е� ���;��I����;��II��������������������� (���һ��Ϳ�)��
��2����ͼ����KMnO4��Ũ���ᷴӦ��ȡ���������ļ���װ�á�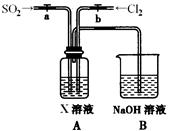
��֪��2 KMnO4+16HCl��Ũ��= 2KCl+2MnCl2+5Cl2��+8H2O��װ��B��C��D�����÷ֱ��ǣ�
B�� ��
C�� ��
D�� ��
��3��ijͬѧ���������װ��̽���������������Ļ�ѧ���ʡ�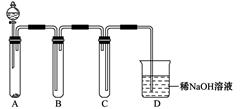
ͨ�����Ƶ��ɼ�a��b����װ��A�зֱ�ͨ�벻ͬ���壬������������⣺
�����ر�b����a����XΪƷ����Һ����A�е�����Ϊ�� ��˵������������� �ԡ�
�����ر�a����b����XΪ��ɫʯ����Һ����A�е�����Ϊ�� ��ԭ���� ��
����ͬʱ��a��b����ͨ������������Ϊ1:1����XΪ��ɫʯ����Һ����A�е�����Ϊ ���������ͬ��ԭ���� ����д��Ӧ�Ļ�ѧ����ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧѧϰС��ͬѧ����ʵ�������е���ȡ������ҩƷ���������ͼ��ʾ��ʵ��װ�ã����ּг�����δ����������ȡ��̽�������Ļ�ԭ�ԡ����鷴Ӧ�����ش��������⣺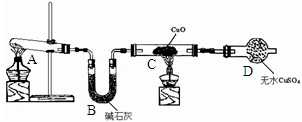
��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2��B�м�ʯ�ҵ������� ��
��3��C�к�ɫ�����죬�Ҳ���������Կ�������Ⱦ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
D�����������________________________________________________________________________��
��4����װ�ô�������ȱ�ݣ���ȱ���� ��
��5����ҵ�г��õ����������ڸ��¡���ѹ������ý�������������ºϳɰ�������С��ͬѧģ�������Ҳ�ϳɳ��˰�������֪��ʼʱ����2 mol N2��6 mol H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ������5 min�������������ʵ���������1 mol���������ʱ������H2��ʾ�Ļ�ѧ��Ӧ����
Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
I��������������Ҫ�Ĺ�ҵԭ�ϣ�̽�����Ʊ����������ʾ��зdz���Ҫ�����塣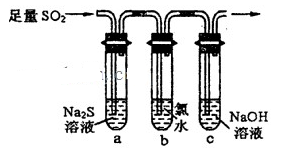
��1����ҵ���û�����FeS2������SԪ��Ϊ��l�ۣ��ڸ����º�������Ӧ�Ʊ�SO2�� ���÷�Ӧ�б�������Ԫ����________����Ԫ�ط��ţ���
���÷�Ӧ�б�������Ԫ����________����Ԫ�ط��ţ���
��2��һ��ѧ�о���ѧϰС�����������װ����֤��������Ļ�ѧ���ʣ�
����˵������������������Ե�ʵ������Ϊ________________________________��
��Ϊ��֤��������Ļ�ԭ�ԣ���ַ�Ӧ��ȡ�Թ�b�е���Һ�ֳ����ݣ��ֱ��������ʵ�飺
���������һ����Һ�м���AgNO3��Һ���а�ɫ��������
��������ڶ�����Һ����Ʒ����Һ����ɫ��ȥ
���������������Һ����BaC!����Һ��������ɫ�������������������Ƿ���______������������Թ�b�з�����Ӧ�����ӷ�Ӧ����ʽΪ____________________��
�۵�ͨ������������Թ�c����Һ������ʱ����Һ��c��Na+����________________���ú���Ԫ����Ũ�ȵĴ���ʽ��ʾ����
����һ��ѧ�о���ѧϰС����ʵ����������������ͭ��ҺΪ���Һ���õ��ķ���ʵ���˴�ͭ���ᴿ������������͵��Һ�н������л��պͺ����ⶨ����֪��ͭ�к���������п������������Ƚ����������������ʣ������Ӧ����
����һ����⾫�ƣ�
���ʱ����ͭӦ���Դ��______�������������ϵĵ缫��ӦʽΪ____________��
������������ɺ�С��ͬѧ���������̶Ե��Һ���д�����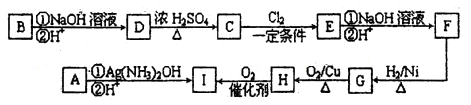
ϡ���ᴦ��������õ�������ϡ��Һ����д���÷�Ӧ�����ӷ���ʽ��
____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������ϡ������ͭ��Ӧ���Ʊ�NO���壬������ã���)װ�ã���ƿ�ڼ���ϡ�����ͭƬ����Ҫʱ�ɼ��ȣ�,ʵ��Ч����ʮ�����룬��Ϊ�ӹ۲쵽������������֤����Ӧ������NO����������ˣ���)װ�ã���Ƥ���¶�����ͭ˿Ȧ������������ʵ����Դﵽ�����Ч��������Ҫ��ش��������⣺
��1���â�װ����ʵ��ʱ��ʵ����������ڹ۲쵽��ƿ���� ������������֤����Ӧ������NO���ռ�NO�ܷ���ƿ�������ſ��������� ����ܡ�������
��2���â�װ����ʵ��ʱ�����йز���������ȫ��
�ٽ���Һ©���Ļ�������U�ιܵ�B��ܿ�ע��ϡ���ᣬһֱע�� Ϊֹ��
a���պý�ûͭ˿�¶� b���պý�ûͭ˿�в� c�� ������������Һ��䲻���п�϶
�ڹرջ������þƾ��ƶ�U�ιܵ�A����ȣ��� ʱ����ȥ�ƾ��ơ�
��3���ڣ�2����ʵ���У�
��ʲô����·�Ӧ�Զ�ֹͣ�� ��
�δ��۲쵽��ɫ��NO���壿 ��
������ٽ���Һ©���Ļ������������ڷ�Һ©���й۲쵽��Щ��������
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ף��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ����������ʵ�����̣�
��ͼA��B��CΪ�ף�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��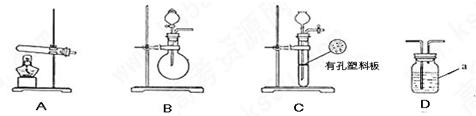
ʵ�鿪ʼǰװ���еĿ������ž�����С���ã���Ӧǰ����ͭ������Ϊ ������ͭ��Ӧ��ʣ����������Ϊ
������ͭ��Ӧ��ʣ����������Ϊ �����ɵ����ڱ�״���µ����
�����ɵ����ڱ�״���µ����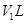 ����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
��1��д������a�����ƣ� ��
��2���ף�����С��ѡ���˲�ͬ������ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��С�
| | ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� |
| ��С�� | A | �������ƣ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� |
| ��С�� | �� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ�����ȱ�����ɫ������
| A��SO2����ɫʯ����Һ | B��������Cl2��KI������Һ |
| C�����������Ǧ��ֽ | D��Na2O2���̪��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com