
 2HI(g) ��H����9.48 kJ��mol-1
2HI(g) ��H����9.48 kJ��mol-1  2HI(g) ��H����26.48 kJ��mol-1
2HI(g) ��H����26.48 kJ��mol-1| A��254g I2(g)��ͨ��2g H2(g)����Ӧ����9.48 kJ |
| B������Ӧ������52.96kJ����ʱת��2moleһ |
| C����Ӧ�ڵķ�Ӧ���������ȷ�Ӧ�ٵķ�Ӧ���������� |
| D��1 mol��̬����1 mol��̬�������������17.00 kJ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2H2O(g) �� 2H2(g) �� O2(g)��H1 2H2O(l) �� 2H2(g) �� O2(g)��H2 |
| B��S (g) �� O 2(g) �� SO2(g)��H1 S (s) �� O 2(g) �� SO2(g)��H2 |
| C��2C (s) �� O 2(g) �� 2CO (g)��H1 2C (s) �� 2O 2(g) �� 2CO2 (g)��H2 |
| D��H2(g) �� Cl2(g) �� 2HCl(g)��H1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����H1����H2����H3����H4 | B����H1����H2����H3����H4 |
| C����H1����H2����H3����H4 | D����H1����H2����H3����H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
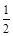 O2(g) ��CO2(g)����H����283.0 kJ��mol-1
O2(g) ��CO2(g)����H����283.0 kJ��mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��-386kJ��mol-1 | B��+386kJ��mol-1 | C��- | D��+746kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��S��ȼ����Ϊ297��23kJ��mo1 |
| B��S(g)+O2(g)===SO2(g)�ų�����������297��23kJ |
| C��S(g)+O2(g)==SO2(g)�ų�������С��297��23kJ |
| D���γ�1mol SO2(g)�Ļ�ѧ�����ͷŵ����������ڶ���1molS(s)��lmolO2(g)�Ļ�ѧ�������յ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Q1+Q2+Q3 | B��0.5(Q1+Q2+Q3) | C��0.5Q1��1.5Q2+0.5Q3 | D��1.5Q1��0.5Q2+0.5Q3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com