
���� ȼ�պ�����һ����̼��˵���������㣬��ȫ��Ӧ������Ũ�������ؿ�֪ˮ�����������ݼ�ʯ�����ؿ�֪������̼�����������������غ㶨�ɿ�֪һ����̼���������������2.3gA��C��H��O��ԭ�Ӹ������Լ�A�ķ���ʽ���Դ˽����⣮
��� �⣺n��O2��=$\frac{2.8L}{22.4L/mol}$=0.125mol��m��O2��=0.125mol��32g/mol=4g��
���������غ㶨�ɿɵã�m���л��+m��O2��=m��CO��+m��CO2��+m��H2O����
��m��CO��=2.3g+4g-2.7g-2.2g=1.4g��
������Ԫ�������غ�ɵ�2.3gA��O���������ڲ�������Ԫ�ص�������-������������
��$\frac{1.4g}{28g/mol}��16g/mol$+$\frac{2.2g}{44g/mol}��32g/mol$+$\frac{2.7g}{18g/mol}��16g/mol$-4g=0.8g��
��1��2.3gA��n��C��=$\frac{2.2g}{44g/mol}$+$\frac{1.4g}{28g/mol}$=0.1mol��
n��H��=2n��H2O��=2��$\frac{2.7g}{18g/mol}$=0.3mol��
��2.3g A��������ԭ�ӡ�̼ԭ�ӵ����ʵ�������0.3mol��0.1mol��
��2��2.3gA��n��C��=$\frac{2.2g}{44g/mol}$+$\frac{1.4g}{28g/mol}$=0.1mol��
n��H��=2��$\frac{2.7g}{18g/mol}$=0.3mol��
n��O��=$\frac{0.8g}{16g/mol}$=0.05mol��
����2.3gA��C��H��O��ԭ�Ӹ�����Ϊ0.1mol��0.3mol��0.05mol=2��6��1��
��ʵ��ʽΪC2H6O���ﵽ���ͣ������ʽΪC2H6O��
�𣺸��л���ķ���ʽΪC2H6O��
��3������ʽΪC2H6O����Ӧ�Ľṹ��ʽ����ΪCH3CH2OH��CH3OCH3���ܺ��Ʒ�Ӧ����������ӦΪCH3CH2OH��
���л�����ܵĽṹ��ʽΪCH3CH2OH��
���� ���⿼���л������ʽ��ȷ����Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬��Ŀ�Ѷ��еȣ�ע��������غ�ĽǶȽ�������Ŀ��


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.8molNaCl���������� | B�� | ��״����5.6L CO2����ԭ���� | ||
| C�� | 1L1 mol•L-1������Һ����Hԭ���� | D�� | 10g��������ԭ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ʒ�� | Ũ������֭ |
| ���� | ˮ��Ũ������֭�����ǡ������ᡢ�����ء�ά����C�������㾫�����ʻơ�����ơ�ɽ����صȣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCl��HBr��HI��HF�ķе��������� | |
| B�� | ��������N-H���ļ���С��ˮ������O-H���ļ��� | |
| C�� | �Ҵ����Ӽ�����������ȩ���Ӽ䲻������������Ҵ��ķе������ȩ | |
| D�� | ����N-H��ǿ��P-H������NH3�ķе����PH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ϡ�����м�һ����CaCO3����������̼��Ƶ�������������Ϊ��������� | |
| B�� | Ca��OH��2��Һ�е�����Һ����������Na2CO3��Һ�������������Ϊ���������� | |
| C�� | ϡ����μӵ�AgNO3��Һ�У���������ϡ����������������Ϊ��Һ�ĵ��������� | |
| D�� | ϡ����μӵ�Ba��OH��2��Һ�У���������ϡ����������������Ϊ��Һ�ĵ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 12�� | B�� | 24�� | C�� | 48�� | D�� | 96�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
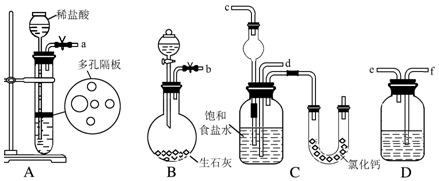
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com