
(1)���ڵ��۵⻯����Һ�У��μ������������Ƽ�����Һ�������ῴ����Һ����ɫ��������Ϊ________________________________________________________��
���ӷ���ʽΪ_________________________________________________________��
���ڵ�͵����γɵ���ɫ��Һ�У��μ��������Ƽ�����Һ��������ɫ����ʧ��������Ϊ________________________________________________________________________��
���ӷ���ʽ��____________________________________________________________��
�۶ԱȢٺ͢�ʵ�����õĽ������I2��ClO����SO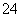 ����������ǿ������˳������Ϊ____________��
����������ǿ������˳������Ϊ____________��
(2)���ʵ��֤��������ʵ��д����ѧ��Ӧ����ʽ��
��Ũ����������Ա�ϡ����ǿ��
���Ȼ�����Һ��Fe3���������Ա�����ͭ��Һ�е�Cu2��ǿ��
�����Ļ�ԭ�Ա�ͭǿ��
������(1)�������ƾ���ǿ�����ԣ�������I�����ɵ���I2��I2�����۱�����Na2SO3���л�ԭ�ԣ��ɻ�ԭI2����I����ʹ��ɫ��ʧ�����ӷ���ʽ����д˼·�ǣ�ȷ����Ӧ����Ȼ����ݵ��ӵ�ʧ�غ�͵���غ���ƽ��
(2)�ٿɸ���Cu��ŨH2SO4���ȷ�Ӧ����Cu��ϡH2SO4����Ҳ����Ӧ��֤�����ڢۿ�����������ԭ����ʽ��֤����
�𰸣�(1)��NaClO��KI����������I2
ClO����2I����H2O===I2��Cl����2OH��
��I2��Na2SO3��ԭ������I��
SO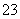 ��I2��2OH��===SO
��I2��2OH��===SO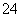 ��2I����H2O
��2I����H2O
��ClO��>I2>SO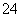
(2)��Cu��ŨH2SO4�ڼ���ʱ��Ӧ����ϡH2SO4�ڼ���ʱ����Ӧ
Cu��2H2SO4(Ũ)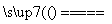 CuSO4��SO2����2H2O
CuSO4��SO2����2H2O
��Cu��FeCl3��Һ�ܷ�Ӧ
2FeCl3��Cu===2FeCl2��CuCl2
��Fe����CuSO4��Һ��Ӧ�û���Cu
Fe��CuSO4===FeSO4��Cu(��������)



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
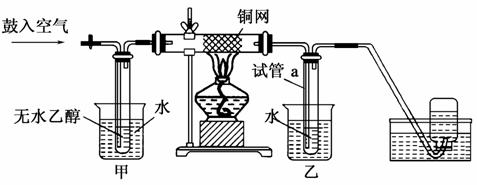
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ��___________________________________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ���������Ӧ��________��Ӧ��
(2)��������ˮԡ���ò���ͬ��
��������________���ҵ�������________��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������________������ƿ���ռ������������Ҫ�ɷ���________��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���________(��д��ĸ)��
a���Ȼ�����Һ b����
c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ��________(��ʵ���������)���ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25 ��ʱ�������й���Һ���������ʵ���Ũ�ȹ�ϵ��ȷ����(����)
A��0.1 mol��L��1CH3COONa��Һ��0.1 mol��L��1HCl��Һ�������ϣ�
c(Na��)��c(Cl��)>c(CH3COO��)>c(OH��)
B��0.1 mol��L��1NH4Cl��Һ��0.1 mol��L��1��ˮ��������(pH>7)��
c(NH3��H2O)>c(NH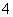 )>c(Cl��)>c(OH��)
)>c(Cl��)>c(OH��)
C��0.1 mol��L��1Na2CO3��Һ��0.1 mol��L��1NaHCO3��Һ�������ϣ�
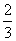 c(Na��)��c(CO
c(Na��)��c(CO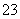 )��c(HCO
)��c(HCO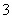 )��c(H2CO3)
)��c(H2CO3)
D��0.1 mol��L��1Na2C2O4��Һ��0.1 mol��L��1HCl��Һ��������(H2C2O4Ϊ��Ԫ����)��
2c(C2O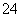 )��c(HC2O
)��c(HC2O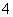 )��c(OH��)��c(Na��)��c(H��)
)��c(OH��)��c(Na��)��c(H��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��
�����ԣ�IO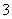 >Fe3��>I2
>Fe3��>I2
��ԭ�ԣ�S2O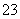 >I��
>I��
3I2��6OH��===5I����IO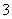 ��3H2O��KI��I2
��3H2O��KI��I2 KI3
KI3
(1)ȡһ����ij�ӵ���(���ܺ��� KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ�� ����Һ�м�����KI���壬��Һ�Ե���ɫ���� CCl4 ��ȡ���²���Һ���Ϻ�ɫ����Ӧ�����ӷ���ʽΪ________________��________________��
����Һ�м�����KI���壬��Һ�Ե���ɫ���� CCl4 ��ȡ���²���Һ���Ϻ�ɫ����Ӧ�����ӷ���ʽΪ________________��________________��
(2)KI ��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��д����ʪ������KI ��������Ӧ�Ļ�ѧ����ʽ��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ijFeBr2��Һ�У�ͨ��1.12 L(��״����)��Cl2�������Һ��c(Br��)��3c(Cl��)��0.3 mol/L����Ӧ��������Һ������仯���ƣ�������˵������ȷ����(����)
A��ԭ��Һ��Ũ��Ϊ0.1 mol/L
B����Ӧ����Һ��c(Fe3��)��0.1 mol/L
C����Ӧ����Һ��c(Fe3��)��c(Fe2��)
D��ԭ��Һ��c(Br��)��0.4 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л����һ�������к���һ����CH3��һ����C6H4(�����ṹ)��������CH2����һ����OHԭ���ţ��������ֽṹ���ж��֣���������NaOH��Һ��Ӧ�����ʹ���(����)
A��3�֡������������������� B��6��
C��9�� D��12��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ձ���ɽ��ѧ���ڱ��ﲩϲ���о�Ա�����µ�ͨ��ʵ�鷢�֣�����Ҷ�ӵ�����ϸ���ܹ������к���ѧ����˫��A�Ķ��ԡ�˫��A�Ľṹ��ʽ����ͼ��ʾ�������йش����ʵ�˵����ȷ����(����)
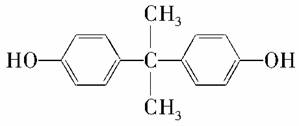
A��1 mol��������������ˮ��Ӧ����2 mol Br2
B������������̼��������Һ��Ӧ�ų�CO2
C�������ʵ�����̼ԭ�ӿ�����ͬһƽ��
D�����������������������ӳɷ�Ӧ���������ʵĻ�ѧʽΪC15H28O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ұ��ҵ�ϣ���������ͨ����ѧ��ԭ���ƵõĽ�������(����)
A��Na��Mg��Al B��Na��K��Zn��Fe
C��Zn��Fe��Cu��Ag D��Mg��Al��Zn��Fe
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
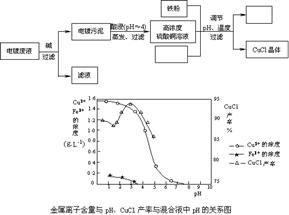
�Ȼ���ͭ(CuCl)���л��ϳɹ�ҵ��Ӧ�ýϹ㷺�Ĵ��������ǰ�ɫ��ĩ������ˮ���������Ҵ����ڿ����лᱻѸ�������������Ե�Ʒ�Һ(��Ҫ��Cu2+��Fe3+)���Ʊ��Ȼ���ͭ�Ĺ�������ͼ���£�
��ش��������⣺
(1)����������Ҫ�ɷ��� (д��ѧʽ)��
(2)���ʱ������Ӧ�����ӷ���ʽ�� ��
(3)����CuCl����ʱ�����pH�� ���ң�
(4)���ۡ��Ȼ��ơ�����ͭ����Һ�з�Ӧ����CuCl�����ӷ�Ӧ����ʽΪ��
��
(5)������CuCl����Ҫ��������ˮ�Ҵ�ϴ�ӣ�Ȼ����ո����ȴ���ܷ��װ����ո���ܷ��װ��Ŀ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com