
Ϊȷ��ij���ȼ�(������������)����ɣ��ֱ��������ʵ�顣
(1)��ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ�����(��״������ͬ)���Ϊb L��
��Ӧ�Ļ�ѧ����ʽ��________________����Ʒ������������________g��
(2)��ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��__________________________����������������������______��
(3)��(2)�з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L����������(1)����������������cb��________��
�𰸡�(1)2Al��2NaOH��2H2O===2NaAlO2��3H2��
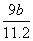
(2)2Al��Fe2O3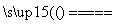 Al2O3��2Fe��80:27
Al2O3��2Fe��80:27
(3)2: 3
������(1)����Ʒ����������Ϊx������
2Al��2NaOH��2H2O===2NaAlO2��3H2��
2��27 g 3��22.4L
x �� bL
x��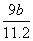 g
g
(2)����Ʒǡ����ȫ��Ӧ������2Al��Fe2O3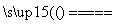 Al2O3��2Fe��֪m(Fe2O3)m(Al)��160(2��27)��8027��
Al2O3��2Fe��֪m(Fe2O3)m(Al)��160(2��27)��8027��
(3)��(2) �з�Ӧ����ʽ����ӦFe��2HCl===FeCl2��H2�����ɵù�ϵʽ��
Al������Fe������H2
27 g 22.4 L
x cL
x��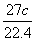 g
g
������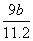 g��
g��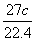 g
g
C:b��2:3��


 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ũ������е�������(����)
A�������ԡ��������������� B������
C����ˮ�� D���ӷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�����֬��������ȷ����(����)
A�����͡����͡����͡�ʯ��������֬�е�����
B�����͡������͡�ţ�Ͷ���������
C���������ͣ�������֬��
D����֬�������γɵ�������֬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���ͨ���Ӿ۷�Ӧ���ɸ߾������ˮ�����������л���������л���Ľṹ��һ���߱��Ļ�����(����)
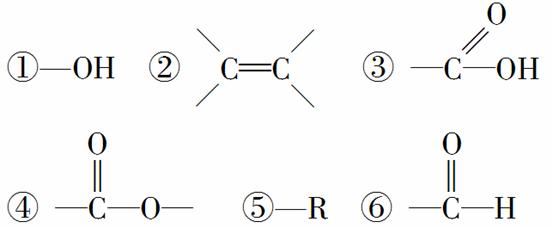
A���٢ڢ� B���ڢۢ�
C���ڢܢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������У���ȷ����(����)
A������5% NaCl��Һʱ������ȷ������NaCl�����ձ��в���������ˮ�����ܽ�
B������1 mol��L��1 NaOH��Һʱ�����ܽ���NaOH��Һ����ע������ƿ
C������0.1 mol��L��1��H2SO4��Һʱ������ȡ��ŨH2SO4��������ƿ�м�ˮϡ��
D������1 mol��L��1 Na2CO3��Һ500 mL����Na2CO3����������ƽ���̳���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���У���ȷ����(����)
A�����ǿ��������Ƚ��Ļ�ѧ�������豸�����µ�ԭ��
B�����ǿ������ô���ʹˮ�������
C�����ǿ��������Ƚ��ļ������豸�����µķ���
D����ѧ��ѧֻ��ͨ��ʵ����̽�����ʵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��Y��Z��W���ֶ�����Ԫ�������ڱ��е����λ����ͼ������˵����ȷ���ǣ�������
| X | Y |
| Z | W |
| �� | A�� | �ǽ����ԣ�X��Y��Z |
| �� | B�� | W��ԭ������������Y��ԭ��������2�� |
| �� | C�� | ��̬�⻯���ȶ��ԣ�Y��W |
| �� | D�� | Z�Ļ�ѧ������һ����X ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�����ʷ�������Ҫ����________����Ԫ�ء�
(2)��������Һ����Ũ������淋�������Һ���������������ټ�����ˮ�����������ܽ⣬��Ȼ����ԭ���Ļ��ԡ�������̳�Ϊ________�������������ᡢ��ؽ����Ρ��Ҵ��Ȼ�ѧ����ʱ�ᷢ�����ᣬʧȥԭ���Ļ��ԣ�����仯��Ϊ________��
(3)����ɱ����ԭ��������
________________________________________________________________________��
(4)������ᴿ�����ʵķ�����
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com