
��14�֣� ����ij��̼�Ͻ� (����̼���ֵ��ʵĻ����)��ij��ѧ��ȤС��Ϊ�˲ⶨ��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ã��г�������ʡ�ԣ���ʵ�鷽������ʵ��̽����
I �ⶨ��������������
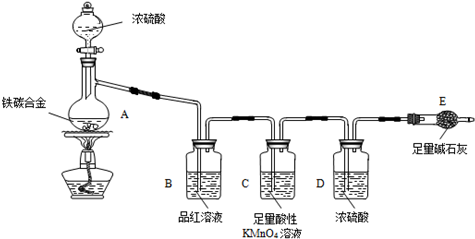
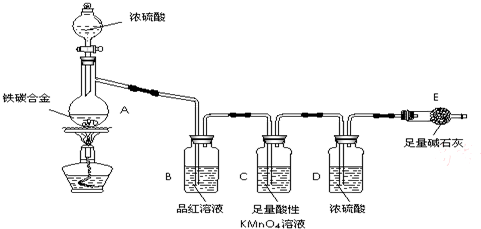
��1���������װ�������Ե�һ�ַ����ǣ��رշ�Һ©���Ļ�������Eװ�ú�����
��һ�����ܣ�Ȼ��__________________________________����֤��װ�õ����������á�
��2������E������������a g��̼�Ͻ���Ʒ����װ��A�У��ټ���������Ũ���ᣬ
��A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����أ�E����b g����̼�Ͻ���������������Ϊ__________________________(д����ʽ)��
��3��װ��C������______________________________________________��
��4����ͬѧ��Ϊ�����ݴ�ʵ���õ����ݣ�����Ͻ������������������ܻ�ƫ�ͣ�
ԭ���ǿ�����CO2��H2O����E��ʹb��������Ϊ�Ľ��ķ�����___________________________________________________________��
��5����ͬѧ��Ϊ����ʹ��ͬѧ��Ϊ��ƫ��õ��Ľ������ݴ�ʵ���úϽ�����
����������Ҳ���ܻ�ƫ�ߡ�����Ϊ���е�ԭ����_________________��
�� ̽��Ũ�����ijЩ���ʣ�
��6����A�еμ�������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ�_______________________________________________________��
��7��A������Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽ��_______________________��
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| ||
| ||
| 11m-3b |
| 11m |
| 11m-3b |
| 11m |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡ̫ԭ���и���5���¿������ۣ���ѧ���� ���ͣ�ʵ����
��18�֣�����ij��̼�Ͻ�ij��ѧ��ȤС��Ϊ�˲ⶨ��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ú�ʵ�鷽�����г�������ʡ�ԣ����������������ش���Ӧ���⡣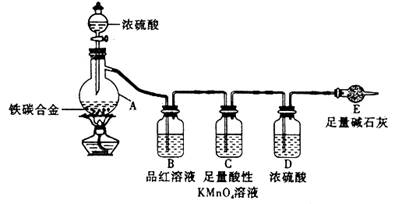
��̽��Ũ�����ijЩ����
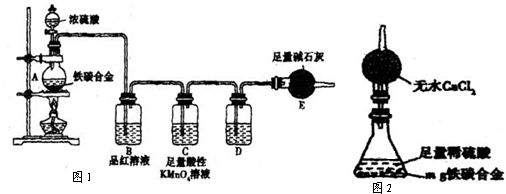
��1����ͼʾ������װ�ã����װ�õ������ԣ�����E��������
��2����a g��̼�Ͻ���Ʒ����A�У��ټ���������Ũ���ᡣ����A������Ϊ________��
δ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ�____ ��
��3����ȼ�ƾ���һ��ʱ���A��B�пɹ۲쵽���Ե�����
д��A�з�����Ӧ�Ļ�ѧ����ʽ___________________________(ֻдһ��)��
B�е�������______________,�ɴ˿ɵõ�Ũ�������____________�ԡ�
��4�����ŷ�Ӧ�Ľ��У�A�л����ܷ���ijЩ���ӷ�Ӧ��д����Ӧ�����ӷ���ʽ________________________________(ֻдһ��)��
��5����Ӧһ��ʱ���A���ݳ������������Ȼ�Ͽ죬�����¶Ƚϸߣ���Ӧ�����⣬�����ܵ�ԭ����_____________________________________��
��ⶨ������������
��6����A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����ء�E����b g��
��̼�Ͻ���������������Ϊ_____________________(д����ʽ)��
��7��ijͬѧ��Ϊ���������ϸ��ӣ�ʹ����ͼ��ʾ��װ�ú���������ʵ�������ⶨijЩ���ݼ��ɡ�Ϊ�˿��ٺ�ȷ�ļ�����������������������ʵ�������_________________(��д����)��
A������ˮ���ⶨH2�����
B����Ӧ�������ˡ�ϴ�ӡ������������������
C���ⶨ��Ӧǰ��װ�ú�ҩƷ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com