
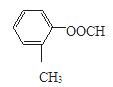
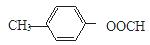
����ѧ—ѡ��5���л���ѧ������A��һȡ�����㻯�����Է�������Ϊ136��������ֻ��̼���⡢�����������ĺ���Ϊ23.5%��ʵ������� A�ķ���������ֻ��һ�������ţ�A��NaOH��Һ��Ӧ���ữ���Եõ�E��C7H6O2����F��
��1��A��E��F�Ľṹ��ʽ��
��2��A��NaOH��Һ��Ӧ���ữ��ʵ��װ�����£�
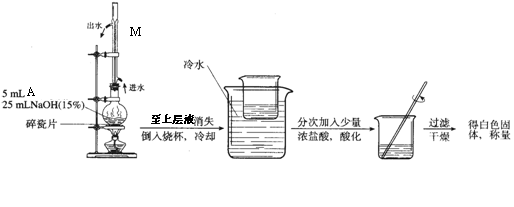

 д��A��NaOH��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽ ��
д��A��NaOH��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽ ��
д��ʵ��װ����M�����ƺ����� ��
��3��A�ж���ͬ���칹�壬�������������Ľṹ���� �֣�
�ٿ��Է���������Ӧ �����ڷ����廯������߱�������״�ṹ
�ۿ���������������Һ��Ӧ �ܲ�����FeCl3������ɫ��Ӧ
��д�����к˴Ź���������5�����շ��A�Ľṹ�Ľṹ��ʽ��
��֪ʶ�㡿����ˮ�� ����ͬ���칹�����дK1 L6
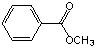
���𰸽�������1��A�Ľṹ��ʽ��
 ��
�� 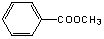
E�Ľṹ��ʽ�� C6H5COOH �� F�Ľṹ��ʽ�� CH3OH ��
��2��C6H5COOCH3+NaOH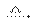 C6H5COONa+CH3OH�������ܡ�����������
C6H5COONa+CH3OH�������ܡ�����������
��3�� 4�� 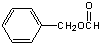
������A��Է�������Ϊ136���������ĺ���Ϊ23.5%�����Է����е���ԭ�Ӹ���Ϊ��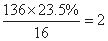 �����A��һȡ�����㻯���������ֻ��̼���⡢����A�ķ���������ֻ��һ�������ţ����ɵõ�A�Ľṹ�ǣ�
�����A��һȡ�����㻯���������ֻ��̼���⡢����A�ķ���������ֻ��һ�������ţ����ɵõ�A�Ľṹ�ǣ�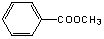 ��A��NaOH��Һ��Ӧ���ữ���Եõ�E��C7H6O2����F����������ˮ���֪E�DZ����C6H5COOH ��F�Ǽ״���CH3OH ��
��A��NaOH��Һ��Ӧ���ữ���Եõ�E��C7H6O2����F����������ˮ���֪E�DZ����C6H5COOH ��F�Ǽ״���CH3OH ��
��2���������ڼ���������ˮ��Ϊ�����κʹ���C6H5COOCH3+NaOH C6H5COONa+CH3OH��װ����M�������������ܣ�����������������
C6H5COONa+CH3OH��װ����M�������������ܣ�����������������
��3��ͬʱ����4��������ֻ���Ǽ��������ֱ��ǣ�
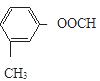
 ��4�֣����к˴Ź���������5�����շ��A�Ľṹ�Ľṹ��ʽ��
��4�֣����к˴Ź���������5�����շ��A�Ľṹ�Ľṹ��ʽ�� ��
��
��˼·�㲦�����⿼��������ˮ�⣬������ͬ���칹�����д��ע������������������


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������[��֪N2(g)��3H2(g)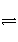
 2NH3(g)����H����92.4 kJ��mol��1]��
2NH3(g)����H����92.4 kJ��mol��1]��
| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1 mol N2�� 3 mol H2 | 2 mol NH3 | 4 mol NH3 |
| NH3��Ũ�� (mol��L��1) | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�a kJ | ����b kJ | ����c kJ |
| ��ϵѹǿ(Pa) | p1 | p2 | p3 |
| ��Ӧ��ת���� | ��1 | ��2 | ��3 |
����˵����ȷ����(����)
A��2c1>c3����������������B��a��b��92.4
C��2p2<p3 D����1����3>1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������CO2����ͨ��NaOH��Ba(OH)2��Na[Al(OH)4]�Ļ����Һ�У���
�ɳ�����ͨ��CO2�����Ĺ�ϵ�ɱ�ʾΪ
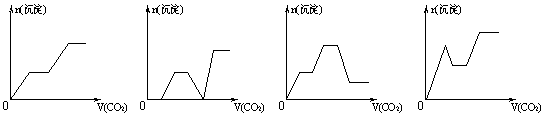 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӧ��2SO2(g)��O2(g)
 2SO3(g)��һ��ʱ���SO3��Ũ��������0.4 mol��L��1�������ʱ������O2��ʾ�ķ�Ӧ����Ϊ0.04 mol��L��1��s��1�������ʱ��Ϊ(����)
2SO3(g)��һ��ʱ���SO3��Ũ��������0.4 mol��L��1�������ʱ������O2��ʾ�ķ�Ӧ����Ϊ0.04 mol��L��1��s��1�������ʱ��Ϊ(����)
��������������������������������������
A��0.1 s B��2.5 s C��10 s D��5 s
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�����������ij�����½���þ�����ֱ��������н���������Ӧʱ���ڽ�����������������Ĥ��ʵ���¼��a��b��Ϊ���¶��йصij�����
| ��Ӧʱ��t/h | 1 | 4 | 9 | 16 | 25 |
| MgO���y/nm | 0.05a | 0.20a | 0.45a | 0.80a | 1.25a |
| NiO���y��/nm | b | 2b | 3b | 4b | 5b |
(1)�����ڸ����µ�������ʴ���ʿ����ý�������Ĥ��������������ʾ����������________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(2)��������Ĥ��Ĥ��y��ʱ��t�����ֵĹ�ϵ�ǣ�MgO����Ĥ��Ĥ��y����________�ͣ�NiO����Ĥ��Ĥ��y��������________�͡�(�ֱ�ߡ��������ߡ���˫���ߡ�������������)
(3)Mg��Ni��Ƚϣ�����________���и��õ���������ʴ�ԣ���������
________________________________________________________________________
______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(�л���ѧ����)�佺��һ����Ȼ����ҩ�һ��������ֻ�ķ�Ⱥһ��ֻ�ܲ�100��˷佺�����Է佺�ֱ���Ϊ����ɫ�ƽ𡱡��佺����Ҫ���Գɷ�Ϊ�����ᱽ����������ӽṹ����ͼ��ʾ(���߱�ʾ��ѧ�����絥����˫����)����һ���������ܷ�������ת����
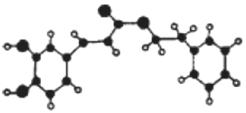
��ش���������:
(1)�����ᱽ�����ķ���ʽΪ .C�Ľṹ��ʽΪ
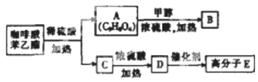
(2)��A�к��������ŵ�����Ϊ �� ��
��A���Է����ķ�Ӧ�� ��������)��
A�� �ӳɷ�ӦB��������ӦC����ȥ��ӦD��������Ӧ
(3)�߷���E�Ľṹ��ʽ�� ��
(4)C��D������Ӧ�ķ�Ӧ������ ��
(5)B��ͬ���칹���кܶ��֣�����ͬʱ��������������ͬ���칹��Ľṹ��ʽΪ ��
a��������ֻ������ȡ����b.�ܷ���������Ӧ c����������������Һ��Ӧ
d���������Ȼ�����Һ������ɫ��Ӧ e���˴Ź�������ͼ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A�����������ʺܻ��ã�����������Ϻ�����������ը
B������������������Һ����ʵ������ȡ����ʱ���������
C������Cl2�������Ƿ����HCl�����ǽ�����ͨ����������Һ
D����ȥCl2�����е�HC l���ɽ�����ͨ�뱥
l���ɽ�����ͨ�뱥 ��ʳ��ˮ
��ʳ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�A�ǿ�����������ˮ���������Ļ���ɫ�������壬A��B��C��D��E����XԪ�أ���ת����ϵ��ͼ��ʾ��
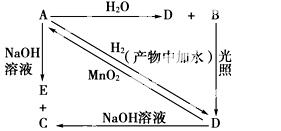
��1����ֱ�д��A��B��D�Ļ�ѧʽ(��Ϊ��Һ�������ʵĻ�ѧʽ)��
A________��B________��D________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ(��ע������)��
A��H2O(���ӷ���ʽ)��_________________________________________________��
A��NaOH(���ӷ���ʽ)��________________________________________________��
D��A(��ѧ����ʽ)��__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������û�������������������ų��ķ�������Ҫ��ѧ�ɷ�ΪSiO2(Լ45%)��Fe2O3(Լ40%)��Al2O3(Լ10%)��MgO(Լ5%)��ijͬѧ��������·�����������Ʒ�и��ֽ���Ԫ�ء���ش��������⡣
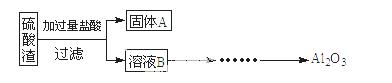
��1��д����ҺB�������� ��
��2���������¿�ͼ��ʽ��һ���� �ɡ���ҺC������Al2O3�������̣�ע���Լ��������Ͳ����� ��
�ɡ���ҺC������Al2O3�������̣�ע���Լ��������Ͳ����� ��
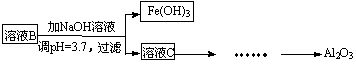
��3��Ϊ�˷���ij����������Ԫ�صĺ������Ƚ�������Ԥ����������Ԫ�ػ�ԭ��Fe2+������KMnO4����Һ�����������½���������ԭ�ζ���д����Ӧ���뷽��ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com