
 ��1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ���100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4Cl���壬��ˮ�ĵ���ƽ���Ӱ���Ǵٽ�����ٽ����������ơ���Ӱ�족����
��1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ���100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4Cl���壬��ˮ�ĵ���ƽ���Ӱ���Ǵٽ�����ٽ����������ơ���Ӱ�족����| ��ѧʽ | ����ƽ�ⳣ����25�棩 |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
���� ��1��Kw=c��H+��•c��OH-������ˮ����δٽ�ˮ�ĵ��룻
��2����������Һ�е���غ���������
��������ĵ��볣����������жϣ�Խ��������Ӧ��ˮ��̶�ԽС��
�����ݵ��볣����С������Ӧ���ɲ��
��� �⣺��1��25��ʱ��ˮ��c��H+��=c��OH-��=10-7 mol/L��Kw=c��H+��•c��OH-��=10-14 �����¶����ߵ�100�棬��ˮ��c��H+��=c��OH-��=10-6 mol/L��Kw=c��H+��•c��OH-��=10-12 ��
100��ʱ1mol•L-1 ��NaOH��Һ��Kw=c��H+��•c��OH-��=10-12 ��c��OH-��=1mol/L��ˮ�������c��H+��=1��10-12��NH4Cl�����ܽ����Һ��笠�����ˮ������һˮ�ϰ��������ӣ���Һ�����ԣ�ˮ�ĵ��뱻�ٽ���
�ʴ�Ϊ��1��10-12���ٽ���
��2���ٵ�Ũ�ȵ�CH3COOH��Һ��NaOH��Һ�������ϣ�������Һ�Լ��ԣ�c��H+����c��OH-��������Һ�е���غ�c��H+��+c��Na+��=c��OH-��+c��CH3COO-����c��Na+����c��CH3COO-����
�ʴ�Ϊ������
������ͼ�����ݷ�����������볣�������������̼��������ӣ����Ե�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һˮ��̶ȵ�Ũ�ȵ�Na2CO3��Һ��NaCN��Һ��CH3COONa��Һ����ҺpHΪNa2CO3��Һ�ģ�NaCN��Һ�ģ�CH3COONa��Һ�ģ�
�ʴ�Ϊ��Na2CO3��Һ��NaCN��Һ��CH3COONa��Һ��
����NaCN��Һ��ͨ������CO2 ��H2CO3���Դ���HCN����HCO3-�����Է�Ӧ���������̼�����ƣ��������ɶ�����̼����Ӧ�Ļ�ѧ����ʽΪ��NaCN+H2O+CO2=HCN+NaHCO3��
�ʴ�Ϊ��NaCN+H2O+CO2=HCN+NaHCO3��
���� ���⿼����������ʵ���ƽ��ķ����жϡ�����ˮ��Ӧ�á���Һ����Եļ����жϣ�ע��̼���Ƕ�Ԫ����ֲ����룬�ڶ��������ƽ�ⳣ��������С����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
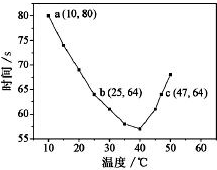 NaHSO3��Һ�ڲ�ͬ�¶��¾��ɱ�����KIO3��������NaHSO3��ȫ���ļ���I2����������I2��������ʱ��������NaHSO3�ķ�Ӧ���ʣ���Ũ�Ⱦ�Ϊ0.020mol•L-1NaHSO3��Һ�����������ۣ�10.0mL��KIO3��������������Һ40.0mL��ϣ���¼10��55�����Һ����ʱ�䣬55��ʱδ�۲쵽��Һ������ʵ������ͼ����ͼ�����������жϲ���ȷ���ǣ�������
NaHSO3��Һ�ڲ�ͬ�¶��¾��ɱ�����KIO3��������NaHSO3��ȫ���ļ���I2����������I2��������ʱ��������NaHSO3�ķ�Ӧ���ʣ���Ũ�Ⱦ�Ϊ0.020mol•L-1NaHSO3��Һ�����������ۣ�10.0mL��KIO3��������������Һ40.0mL��ϣ���¼10��55�����Һ����ʱ�䣬55��ʱδ�۲쵽��Һ������ʵ������ͼ����ͼ�����������жϲ���ȷ���ǣ�������| A�� | 40��֮ǰ��40��֮����Һ������ʱ�����¶ȵı仯�����෴ | |
| B�� | ��NaHSO3��ȫ����ʱ�����ӷ���ʽΪ��6HSO3-+2IO3-=6SO42-+2I-+6H+ | |
| C�� | ͼ��a���Ӧ��NaHSO3��Ӧ����Ϊ5.5��10-5mol•L-1•s-1 | |
| D�� | �¶ȸ���40��ʱ�����۲���������ʵ���ָʾ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
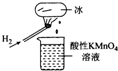 ��ͼ��ʾ��ʵ�飬�����ձ�������KMn04��Һ��ɫ�������ձ��е���Һ���ɺ�������KSCN��FeS04��Һ����Һ��Ѫ��ɫ���ж�����˵���в���ȷ���ǣ�������
��ͼ��ʾ��ʵ�飬�����ձ�������KMn04��Һ��ɫ�������ձ��е���Һ���ɺ�������KSCN��FeS04��Һ����Һ��Ѫ��ɫ���ж�����˵���в���ȷ���ǣ�������| A�� | ��������Hzȼ�������˼Ⱦ����������־��л�ԭ�Ե����� | |
| B�� | ��������H2ȼ�յIJ����п��ܺ���һ������H202 | |
| C�� | ���ձ�����Һ����KI������ҺҲ����֤��������л�ԭ�� | |
| D�� | ����FeSO4��Һ�м���˫��ˮ�����ӷ�ӦΪ��2Fe2++H2O2+2H+=2Fe3++2H20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2CO3��Һ����ʢװ�ڲ��������Լ�ƿ�� | |
| B�� | ����ʳ��ˮʹ�����Ի�ɫ | |
| C�� | FeCl3��Һ�������ɵõ�Fe2O3 | |
| D�� | 0.1mol/LCuCl2��Һ�У�c��Cu2+����0.1mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢ� | B�� | �ۢ� | C�� | �� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com