
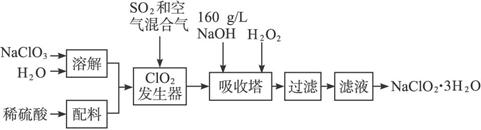
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
�ڴ�ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10�����°�ȫ��
��
(1)
(2)�������й�����������ÿ�����_____________(ѡ�����)��
a.��SO2������SO3����ǿ����
b.ϡ��ClO2�Է�ֹ��ը
c.��NaClO3������ClO2
(3)�������ڵķ�Ӧ�Ļ�ѧ����ʽΪ____________�����������¶Ȳ��ܳ���
(4)�ڼ�����Һ��NaClO2�Ƚ��ȶ���������������Ӧά��NaOH�Թ������ж�NaOH�Ƿ�����ļ�ʵ�鷽����________________________��
(5)��������Ϊ��ֹNaClO2����ԭ��NaCl�����û�ԭ���Ļ�ԭ��Ӧ���С���H2O2�⣬������ѡ��Ļ�ԭ����____________(ѡ�����)��
a.Na2O2 b.Na2S c.FeCl2
(6)����Һ�еõ�NaClO2��3H2O�־����ʵ�����������____________(ѡ�����)��
a.���� b.���� c.���� d.���� e.��ȴ�ᾧ
Ҫ�õ�������NaClO2��3H2O���������еIJ�����____________(���������)��
(1)4 mol��L-1 ����Һ���ܶ�
(2)b
(3)2NaOH+2ClO2+H2O2![]() 2NaClO2+2H2O2+O2 ��ֹH2O2�ֽ�
2NaClO2+2H2O2+O2 ��ֹH2O2�ֽ�
(4)�����ⶨ����������Һ��pH
(5)a
(6)b��e��d �ؽᾧ
������(1)c=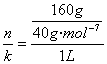 =4 mol��L-1.Ҫ�������Һ����������������Ҫ�õ���Һ������������֪����Һ���Ϊ
=4 mol��L-1.Ҫ�������Һ����������������Ҫ�õ���Һ������������֪����Һ���Ϊ


 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����ģ�⣩��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��Cl
��2013?����ģ�⣩��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��Cl| O | - 3 |
| O | - 3 |
| O | n- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ѹǿMPa ת����% �¶ȡ� |
0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡ��������ͨ���и�����ѧ���������⿼�Ի�ѧ�Ծ� ���ͣ������
��10�֣��������ȼҵ����Ҫ��Ʒ֮һ������һ�ֳ��õ���������������ԭ������ˮ��Ӧ�����˴����
Cl2 + H2O  HCl + HClO K=4.5��10-4
HCl + HClO K=4.5��10-4
�������ǿ��������ɱ��ˮ�еIJ�������ֱ���ô�����Ϊ����ˮ��������Ϊ�������ֽ⣬�Ҷ��Խϴ����ǣ������������˲����㣬�Ҿ���һ����Σ���ԣ�Ŀǰ��������������Խ��������Ʒ���������ش�
��1���ȼҵ���������Ļ�ѧ����ʽΪ ��
��2��84����Һ(��Ҫ�ɷ�ΪNaClO)��������Ⱦ������˷�����ŵ㣬���������ռ���Һ��Ӧ�Ʊ�84����Һ�����ӷ���ʽΪ ��
��3������������һ�ָ�Ч�����ס���ȫ��ɱ�������ʼ����ҹ���ѧ���з��������������������ƣ�NaClO2�������Ʊ��������ȵķ������仯ѧ����ʽΪ ��
��4��һλͬѧ�����һ����Ũ�����KMnO4������ȡ�����������Ƚ�������ⵥ�ʵ���
����ǿ������װ�ã���ͼ����
��������Һ������Cl2���� ������ĸ��ţ���
| A������ʳ��ˮ | B������Na2SO3��Һ |
| C������NaOH��Һ | D��Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡͭ���и������Ĵ��¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪�������Ƶ�����ˮ��Һ��ͨ������������壬��Ӧ��SO2��������Ϊ��SO2+2H2O �C2e�� ��SO42-+4H+������������(NaClO2)������ͨ���ÿ���ϡ�͵���������Ӧ�л�ԭ����Ϊ��Cl2 + 2 e�� ��2Cl����������������Ӧ�о������ɲ���X����X�Ļ�ѧʽΪ�� ��
A��ClO2 B��NaClO4 C��HClO D�� NaClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�����и�����ѧ���������⿼�Ի�ѧ�Ծ� ���ͣ������
��10�֣��������ȼҵ����Ҫ��Ʒ֮һ������һ�ֳ��õ���������������ԭ������ˮ��Ӧ�����˴����
Cl2 + H2O
 HCl
+ HClO K=4.5��10-4
HCl
+ HClO K=4.5��10-4
�������ǿ��������ɱ��ˮ�еIJ�������ֱ���ô�����Ϊ����ˮ��������Ϊ�������ֽ⣬�Ҷ��Խϴ����ǣ������������˲����㣬�Ҿ���һ����Σ���ԣ�Ŀǰ��������������Խ��������Ʒ���������ش�
��1���ȼҵ���������Ļ�ѧ����ʽΪ ��
��2��84����Һ(��Ҫ�ɷ�ΪNaClO)��������Ⱦ������˷�����ŵ㣬���������ռ���Һ��Ӧ�Ʊ�84����Һ�����ӷ���ʽΪ ��
��3������������һ�ָ�Ч�����ס���ȫ��ɱ�������ʼ����ҹ���ѧ���з��������������������ƣ�NaClO2�������Ʊ��������ȵķ������仯ѧ����ʽΪ ��
��4��һλͬѧ�����һ����Ũ�����KMnO4������ȡ�����������Ƚ�������ⵥ�ʵ���
����ǿ������װ�ã���ͼ����

��������Һ������Cl2���� ������ĸ��ţ���
A. ����ʳ��ˮ B. ����Na2SO3��Һ
C. ����NaOH��Һ D. Ũ����
����˵��Cl2��������ǿ��I2��ʵ�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com