
���� ��1���ټӼ�ʹCr3+�γ��ܶȻ���С����������������ȥ��
��1mol Cr2O72-�ķ�ˮ�е�Cr2O72-��ȫת��Ϊһ�ֻ�ѧʽΪCr0.5Fe2.5O4�������壬����ԭ���غ㣬����Cr0.5Fe2.5O4�����ʵ���Ϊ��$\frac{2}{0.5}$=4mol��������������Ҫ�̷���FeSO4•7H2O�������ʵ���Ϊ��4mol��2.5=10mol������m=nM��������⣻Cr0.5Fe2.5O4������ƽ�����ϼ�Ϊ��$\frac{8-0.5��3}{2.5}$=+2.6�ۣ���Fe3+��Fe2+�ĸ���֮��x��y����$\frac{3x+2y}{x+y}=2.6$����֮��0.4x=0.6y���ɴ˷������
��2������2CN-+2ClO2=2CO2��+N2��+2Cl-���м��㣻
��3���ٵζ�ǰ����������������Һ���ɶ�ף����м�ǿ�������ԣ�
�ڶԱ����Ӿ��кܵĻ�ԭ�ԣ��ܻ�ԭ���������۸���
��� �⣺��1���ټӼ�ʹCr3+�γ��ܶȻ���С����������������ȥ���ʴ�Ϊ������NaOH��
��1mol Cr2O72-�ķ�ˮ�е�Cr2O72-��ȫת��Ϊһ�ֻ�ѧʽΪCr0.5Fe2.5O4�������壬����ԭ���غ㣬����Cr0.5Fe2.5O4�����ʵ���Ϊ��$\frac{2}{0.5}$=4mol��������������Ҫ�̷���FeSO4•7H2O�������ʵ���Ϊ��4mol��2.5=10mol������m=nM=10mol��278g/mol=2780g��Cr0.5Fe2.5O4������ƽ�����ϼ�Ϊ��$\frac{8-0.5��3}{2.5}$=+2.6�ۣ���Fe3+��Fe2+�ĸ���֮��x��y����$\frac{3x+2y}{x+y}=2.6$����֮��0.4x=0.6y������x��y=3��2���ʴ�Ϊ��2780��3��2��
��2���������ˮ����Ϊ0.8m3/h�����裨CN-��Ũ��Ϊ300mg/L��1Сʱ��ˮ�к���CN-��������Ϊ0.8��103L��300mg/L=2.4��104mg=24g������CN-�������ʵ���Ϊ$\frac{24g}{26g/mol}$=$\frac{12}{13}$mol������2CN-+2ClO2=2CO2��+N2��+2Cl-��CN-��ClO2��������ClO2�����ʵ���Ϊ$\frac{12}{13}$mol��ʵ����$\frac{12}{13}$��1.3=1.2mol�����������ÿСʱ����Ͷ��ClO2����Ϊ1.2mol��67.5g/mol=81g=0.81kg��
�ʴ�Ϊ��0.81��
��3���ٵζ�ǰ����������������Һ���ɶ�ף����м�ǿ�������ԣ�������ˮ����ɫ���ʣ���ɫ�����ʴ�Ϊ������ˮ����ɫ���ʣ���ɫ����
�ڶԱ����Ӿ��кܵĻ�ԭ�ԣ��ܻ�ԭ���������۸����Է��Ժ�̵ζ��������ţ��ʴ�Ϊ����ˮ�����ܺ��еĸ������Ӽ������λ�ԭ��
���� ������Ҫ�����˹�ҵ��ˮ�ķ��ദ����ע��������ԭ��Ӧ��������ú���صĻ�ѧ֪ʶ�ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaNO3 | B�� | Mg��NO3��2 | ||
| C�� | AgNO3 | D�� | NaNO3��AgNO3�Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{m}{a}L$ | B�� | $\frac{2m}{3a}L$ | C�� | $\frac{m+n}{a}L$ | D�� | $\frac{2��m+n��}{3a}L$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
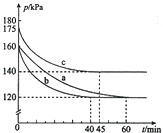 ������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | K+ | Na+ | NH4+ | H+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | a | 4��10-5 | 3��10-5 | 2��10-5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuSO4•3H2O | B�� | CuSO4•2H2O | C�� | CuSO4•H2O | D�� | CuSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 9��4 | B�� | 6��1 | C�� | 7��6 | D�� | 11��6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com