
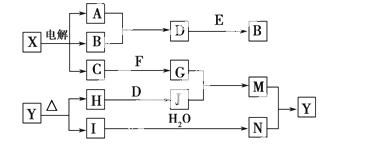
���п�ͼ�е���ĸ�ֱ����һ�ֳ��������ʻ�����Һ���֮���ת����ϵ����ͼ��ʾ(���ֲ��P��Ӧ��������ȥ)����֪A��BΪ��̬���ʣ�F�ǵؿ��к������Ľ���Ԫ�صĵ��ʣ�E��H��IΪ�����EΪ��ɫ���壬IΪ����ɫ���壻MΪ���ɫ������
��ش��������⣺
(1)B������Ԫ�� λ�����ڱ��е�_______
λ�����ڱ��е�_______ _���ڣ�________�塣
_���ڣ�________�塣
(2)A��B��ȼ�յ�������___________________________________________��
(3)D��E����B�ķ�Ӧ�У��������뱻��ԭ�����ʵ����ʵ���֮����________��
(4)G��J����M�����ӷ���ʽ��_______________________________________��
(5)Y���ȷֽ�Ļ�ѧ����ʽ��_______________________________________��
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ����
A����֪C(ʯī, s)=C(���ʯ, s) ��H>0������ʯ��ʯī�ȶ�
B����֪C(s)+O2(g)=CO2(g) ��H1 ��C(s)+1/2O2(g)=CO(g) ��H2����H2>��H1
C����֪2H2(g)+O2(g)=2H2O(g) ��H= -483.6 kJ/mol����������ȼ����Ϊ241.8kJ/mol
D����֪NaOH(aq)+HCl(aq)=NaCl(aq)+H2O(l) ��H=-57.3kJ/mol����20gNaOH��ϡ��Һ��ϡ������ȫ�кͣ��к���Ϊ28.65kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�����һ�������ۺ����۵Ļ������һ�����ijŨ�ȵ�ϡ�����ַ�Ӧ����Ӧ������������ų�����֪����Ļ�ԭ������NH4NO3�����ڷ�Ӧ���������Һ�У���μ���5mol·L��1��NaOH��Һ������NaOH��Һ�����(mL)������ij��������ʵ�����ϵ����ͼ��ʾ����
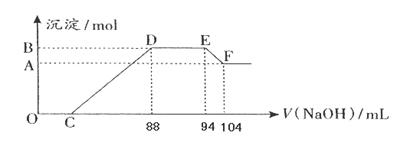
(1)B��A�IJ�ֵΪ�� mol
(2)ԭ������Һ�к���������ʵ���Ϊ�� mol��
(3)���ۺ����۵Ļ���������������۵����ʵ���֮��Ϊ��
(4)д�������Ũ�����ᷴӦ�����ӷ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������з���ȼ�ϵ���ǵ�ǰ���е�һ���ȵ㡣
 ��1����ȼ�ϵ��ʹ�õĵ������Һ��2 mol��L��1��KOH��Һ����ط�ӦΪ��4NH3��3O2 =2N2��6H2O���õ�ظ����ĵ缫��ӦʽΪ ��ÿ����1.7g NH3ת�Ƶĵ�����ĿΪ ��
��1����ȼ�ϵ��ʹ�õĵ������Һ��2 mol��L��1��KOH��Һ����ط�ӦΪ��4NH3��3O2 =2N2��6H2O���õ�ظ����ĵ缫��ӦʽΪ ��ÿ����1.7g NH3ת�Ƶĵ�����ĿΪ ��
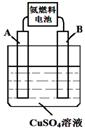
��2���ð�ȼ�ϵ�ص��CuSO4��Һ������ͼ��ʾ��A��B��Ϊ���缫��ͨ��һ��ʱ�����A�缫���к�ɫ������������B�缫�Ϸ����ĵ缫��ӦʽΪ ����ʱ��������Һ�м���8gCuO�����ǡ�ÿ�ʹ��Һ�ָ������ǰ��Ũ�ȣ�����������ռ����������ڱ�״�������Ϊ L��
��3�� ����������ͭ(Cu2O)��һ����Ҫ�����ϡ�����ͭ����ʯī���缫������ʳ��ˮ��������Ʊ�����������ͭ(Cu2O)����ⷴӦΪ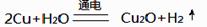 ��
��
ͭ���Ϸ����ĵ缫��ӦʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F����������һ������������ͼZ4��2��ʾ���ת����ϵ�����з�Ӧ�����������Ѹ�����
(1)����Ӧ�١��ڡ��۾�Ϊ��Һ�е��û���Ӧ��A��D��E Ϊ�����Ľ������ʣ��� A ��D ��E �Ļ�ԭ����ǿ������˳��Ϊ________________��
��д����������Ҫ������ӷ���ʽ��
��Ӧ��____________����Ӧ��_____________��
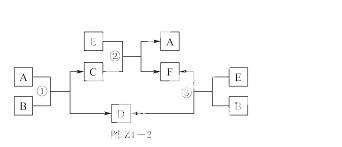
(2)����Ӧ�١��ڡ��۾�Ϊ���ֽⷴӦ����д������Ҫ��Ļ�ѧ����ʽ��
��Ӧ��_ ____________________��
____________________��
��Ӧ��_____________________��
(3)��B��ˮ��C��һ���д��ԵĻ����E��һ����ɫ����ζ���ж����壬��Ӧ�ٵĻ�ѧ����ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D����Ԫ��ԭ�ӵĺ˵������������С��20�����䵥�ʼ���Ӧ�Ļ������ܷ������·�Ӧ��ϵ��

��1��д��F�ĵ���ʽ ��
��2������H�ж�������Na2CO3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��________________��
��3������E��ˮ��Һ�����գ����յõ��Ĺ���Ϊ , ԭ��Ϊ
, ��(�û�ѧ��Ӧ����ʽ����ʾ)
��4�������£���F��ˮ��Һ�м���������Ũ�ȵ����ᷴӦ��������Һ��PH��7�������Һ������Ũ����С�����˳��Ϊ�� ��
��5��������H���ڿ����г��ȼ�տɵõ�����������BO2��BO2��������������Ӧ��2BO2+O2  2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20 mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����
2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20 mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����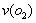 = mol–L-1
= mol–L-1 –min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ������� ֮�䡣
–min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ������� ֮�䡣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʱ���ȥ����Ӧ�ٳ���Ӧ����Ұ�⺸�Ӹֹ죬���ǹ�ҵ����Ҫ�ķ�Ӧ֮һ��

��ش��������⣺
(1)H�ĵ���ʽ��________________�����к��еĻ�ѧ��������___________
_________________��
(2)д����Ӧ�ܵ�����_______________________________________________________________________________________��
�йط�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________________________________��
(3)��֪I��ȼ�����ǣ�285.8 kJ·mol��1����1 m3(��״��)I��ȫȼ�գ��ָ�������ʱ�ų���������________(����������3λ��Ч����)��
(4)25 ��ʱ����PtΪ�缫��⺬���� ����̪��F�ı�����Һ������________(�����������)��������Һ����ɫ��Ϊ��ɫ�����ڴ˼��ռ���0.2 g���壬���ʱ��Һ��pH��________(������Һ�����Ϊ2 L�Ҳ����ǵ�����Һ����ı仯)��
����̪��F�ı�����Һ������________(�����������)��������Һ����ɫ��Ϊ��ɫ�����ڴ˼��ռ���0.2 g���壬���ʱ��Һ��pH��________(������Һ�����Ϊ2 L�Ҳ����ǵ�����Һ����ı仯)��
(5)��K��Һ�м�����K�����ʵ�����Na2O2��ǡ��ʹKת��ΪN��д���÷�Ӧ�����ӷ���ʽ��_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
(1)A�����ڱ��е�λ����________��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ��
__________________________��
(2)B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ__________����ˮ��Һ��F���ʷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________________���ڲ����м�������KI����Ӧ�����CCl4�����л�����______ɫ��
(3)����ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ������A�Ķ�Ԫ���ӻ����� |
| b | �����зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1��1 |
| c | ����ѧ���ΪBDF2 |
| d | ��ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
a�Ļ�ѧʽΪ________��b�Ļ�ѧʽΪ______________��c�ĵ���ʽΪ________��d�ľ���������________��
(4)��A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ��________������ ���п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ__________��
���п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ת���У�A��һ�����Σ�D����Է���������C����Է���������16��E���ᣬYΪ���ʣ���X������ǿ�ỹ��ǿ��ʱ���������µ�ת����ϵ��
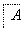
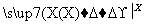
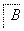
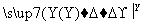
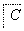
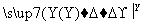
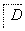
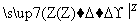

��X��ǿ��ʱ��A��B��C��D��E������ͬһ��Ԫ�أ���X��ǿ��ʱ��A��B��C��D��E����������ͬһ��Ԫ�ء���ش�
(1)A��________��Y��________��Z��________��
(2)��X��ǿ��ʱ��E��________��д��B����C�Ļ�ѧ����ʽ��_______________ _________________________________________________________��
(3)��X��ǿ��ʱ��E��________��д��B����C�Ļ�ѧ����ʽ��_______________ _________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com