
��������������Ⱦ��Ϊ���أ����������������ü�����⡣
��1���������糧Ϊ��ȥ�к�����SO2�������Ϊ����������β�������գ���ͼ��ʾ��д��β���������з�Ӧ�Ļ�ѧ����ʽ�� ��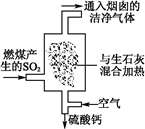
��2�������Ļ������糧ͨ�����ں��ߣ�һ�㺣ˮ�������ԣ���Ҫ����Na+��Mg2+��K+��Ca2+��Cl-��Br-��SO42-��HCO3-�����ӡ�����SO2������Ҳ�������ú�ˮ�����乤����������ͼ��ʾ��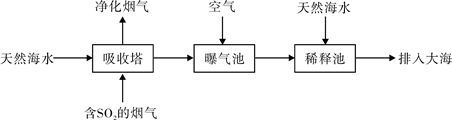
����������ͨ�������Ŀ���� ��
��ͨ���������������еĺ�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ�������� ��
| A��Cl- | B��Na+ | C��Mg2+ | D��HCO3- |
(1) SO2��CaO=CaSO3��2CaSO3��O2=2CaSO4
��2���ٽ�H2SO3��HSO3��������ΪSO42- ��D
(3)SO2��OH��==HSO3 ��
�������������β����������Ҫ�dz�����ȼ�����ɵĶ����������SO2��CaO=CaSO3��2CaSO3��O2=2CaSO4������������������H2SO3��HSO3��������ΪSO42- ͨ���������������еĺ�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ��������HCO3-����������̼������������ȵ�ʱ�����ֽ⡣
���㣺���黯ѧ�������м�����������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ��Ԥ�⣬������ȡ�����ܣ���Ϊ��Ҫ����һ����ɫ��Դ�������й�˵������ȷ����
A��Һ����Ϊ�����Դ�ķ�Ӧԭ����4NH3��5O2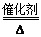 4NO��6H2O 4NO��6H2O |
| B��Һ�����и�ʴ�ԺͶ��ԣ���ʹ�ù�����Ҫ��ֹҺ��й© |
| C�������ȿ����ᣬ��״�����ܶ�ԼΪ0.76 g��L��1 |
| D��������������ȣ��ŵ����ڰ�������������ը��ʹ��ʱ����ȫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ��ʾ������ʢ����Һ�ٺ���Һ�ڵ��Թ��У�ͨ���������X�����բ١��ھ��г������ɵ���
| ѡ�� | X | ����Һ | ����Һ |
| A | Cl2 | Na2SO3 | Na2S |
| B | SO2 | Na2SiO3 | Ba(NO3)2 |
| C | NH3 | AgNO3 | AlCl3 |
| D | HCl | CuSO4 | NaAlO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
N��O��Si��S����Ҫ�ķǽ���Ԫ�أ�����˵����ȷ����
| A��N��O��S��Si��ԭ�Ӱ뾶�����ǽ��������� |
| B��������������������������γɹ⻯ѧ�����������γ��������Ҫ���� |
| C��S��SO2��Si��SiO2�������ʾ�����NaOH��Һ��Ӧ������������ijЩ�ᷴӦ |
| D��N��Si��S�ĵ��ʾ��ܺ�������Ӧ�����ɵIJ���ֱ���NO2��SiO2��SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ����ʱ��������ػ�ɫ�̵��ǣ� ��
| A������������ȼ�� | B��ͭ��������ȼ�� |
| C��������������ȼ�� | D������������ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ���峣��NaBr��ʽ���ڣ���������ˮ�к���0��07%����ˮ�к�NaBr����������Ϊ _________���Ӻ�ˮ����ȡ��ķ���֮һ�ǣ�
ͨ��Cl2��Ũ�����廯����Һ��Ȼ���ÿ��������ɵ��崵����
��Na2CO3��Һ�����壨������5/6��������ʵ���ת��ΪBr������ͬʱ�ų�CO2��
�����ú��廯������Һ�м�ϡH2SO4����������������
�õ������п��ܼ���������Cl2���ټ�������FeBr2��ȥ
��һ����Ӧ�����ӷ���ʽ ____________________________________
���IJ���Ӧ�����ӷ���ʽ�� _________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ҫ�ķǽ���Ԫ�ء�
30.NaCl��Ũ��������ȡ�Ȼ���Ļ�ѧ����ʽΪ ��
���� ��ֽ����ƿ���Լ����Ȼ��������Ƿ�����
31.������ˮ�к��еķ����У�Cl2��H2O�� ������������ˮ�Ļ�ѧ����ʽΪ ����ҵ���õ��ʳ��ˮ��ȡ�����������ĵ缫��ӦʽΪ��
2H++2e��H2��,�������ĵ缫��ӦʽΪ ��
32.��֪��ԭ��SO32-��I-��Br-.��NaBr��NaI��Na2SO3�����Һ�У�ͨ�롪������������Һ���ɲ�������գ��õ�����ʣ�����ʵ���ɿ�����______��ѡ���ţ���
a. NaCl Na2SO4 b. NaCl NaBr Na2SO4
c. NaCl Na2SO4 I2 d. NaCl NaI Na2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣���ͼ���о�ͭ��Ũ����ķ�Ӧװ�ã� 
��1��A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧһ��ʱ��ɹ۲쵽B�Թ��е�����Ϊ ��
��3��C�Թܿڽ���NaOH��Һ������������ ��
��4��ʵ�������֤��A�Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ��������� ��
��5����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣�������������������ϡ�
| ����1 |  ����ͭ��Ũ���ᷴӦ������ɫ���ʵ�������� |
| ����2 | X���߾������������ͭ��Ũ���ᷴӦ���ɵĺ�ɫ����ΪCu2S��CuS��Cu7S4�е�һ�ֻ��֡� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ������֮Դ������ˮ������Ϊ����ˮ�ʵ�һ����Ҫ���ڣ�Һ�����������������ˮ������������������ѧ���������������ˮ�е��л������Ӧ�����ɵ��л��Ȼ�����ܶ������к����������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ����������Cl2��ȣ�ClO2�������и�������ɱ�����������Ҳ��������������DZ��Σ�����л��ȴ��
��1����������ˮ��ɱ��������ԭ���� ��
��2����ClO2���Ʊ������У������������Ʊ�������
����һ��NaClO3��4HCl=2ClO2����Cl2����2NaCl��2H2O
��������C6H12O6+24NaClO3+12H2SO4=24ClO2��+6CO2��+18H2O+12Na2SO4
�÷������Ʊ���ClO2���ʺ���������ˮ������������Ҫԭ���� ��
��3����ClO2������������ˮ��pHΪ5��5~6��5��������һ���������岻��������������ӣ�ClO2������2001���ҹ��������涨������ˮClO2���ĺ���Ӧ������0��2 mg��L��1��
����ˮ��ClO2��ClO2���ĺ��������������������вⶨ��ClO2��I����ԭΪClO2����Cl����ת��������ҺpH�Ĺ�ϵ��ͼ��ʾ����pH��2��0ʱ��ClO2��Ҳ�ܱ�I����ȫ��ԭ��Cl������Ӧ���ɵ�I2�ñ�Na2S2O3��Һ�ζ���2Na2S2O3��I2=Na2S4O6��2NaI
����д��pH��2��0ʱ��ClO2����I����Ӧ�����ӷ���ʽ ��
������Na2S2O3����Һʱ��ʹ�õ���������ƽ��ҩ�ס����������ձ��������⣬����Ҫ��ͼ�е� ������ĸ���ţ���




a b c d e
���������Ӧ��ʵ�鲽�裺
����1��ȷ��ȡVmLˮ�����뵽��ƿ�С�
����2������ˮ����pHΪ7��0~8��0
����3������������KI���塣
����4��������������Һ����c mol��L-1Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV1mL��
����5��������Һ��pH��2��0��
����6������c mol��L-1Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2mL��
�ܵζ��յ�������� ��
�ݸ��������������ݣ���ø�����ˮ���е�ClO2����Ũ��Ϊ mg��L��1���ú���ĸ�Ĵ���ʽ��ʾ����
��4���ж����в�����ClO2����Ũ�Ȳⶨ�����Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�� �������Ʊ���Һ�����У��ձ��е�Na2S2O3��Һ������������ʹ�ⶨ��� ��
�� ���ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬ʹ�ⶨ��� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com