
ĻĀĮŠ»ÆŗĻĪļ£ŗ¢ŁHCl ¢ŚNaOH ¢ŪCH3COOH ¢ÜNH3”¤H2O ¢ŻCH3COONa ¢ŽNH4Cl
£Ø1£©ŹōÓŚČõµē½āÖŹµÄŹĒ £¬ČÜŅŗ³Ź¼īŠŌµÄÓŠ £ØĢīŠņŗÅ£©”£
£Ø2£©³£ĪĀĻĀ0.01 mol/L HClČÜŅŗµÄPH= £»PH=11µÄCH3COONaČÜŅŗÖŠÓÉĖ®µēĄė²śÉśµÄc(OH£) = ”£
£Ø3£©ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾CH3COONaČÜŅŗ³Ź¼īŠŌµÄŌŅņ £¬ĘäČÜŅŗÖŠĄė×ÓÅØ¶Č°“Óɓ󵽊”µÄĖ³ŠņĪŖ ”£
£Ø4£©½«µČPHµČĢå»żµÄHClŗĶCH3COOH·Ö±šĻ”ŹĶm±¶ŗĶn±¶£¬Ļ”ŹĶŗóĮ½ČÜŅŗµÄPHČŌĻąµČ£¬Ōņm n £ØĢī”°“óÓŚ”¢µČÓŚ”¢Š”ÓŚ”±£©”£
£Ø5£©³£ĪĀĻĀ£¬Ļņ100 mL 0.01 mol”¤L£1HAČÜŅŗÖšµĪ¼ÓČė0.02 mol”¤L£1MOHČÜŅŗ£¬Ķ¼ÖŠĖłŹ¾ĒśĻß±ķŹ¾»ģŗĻČÜŅŗµÄpH±ä»ÆĒéæö£ØĢå»ż±ä»ÆŗöĀŌ²»¼Ę£©”£»Ų“šĻĀĮŠĪŹĢā£ŗ 
¢ŁÓÉĶ¼ÖŠŠÅĻ¢æÉÖŖHAĪŖ_______Ėį£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
¢Ś Kµć¶ŌÓ¦µÄČÜŅŗÖŠ£¬
c(M£«)£«c(MOH)= mol”¤L£1”£
£Ø1£©¢Ū¢Ü”¢¢Ś¢Ü¢Ż
£Ø2£© 2 ”¢ 10-3 mol£ÆL
£Ø3£©CH3COO? + H2O  CH3COOH + OH?”¢
CH3COOH + OH?Ӣ
c£ØNa+£©£¾c£ØCH3COO?£©£¾c£ØOH-£©£¾c£ØH+£©
£Ø4£© Š”ÓŚ
£Ø5£©¢Ł Ēæ ¢Ś 0.01
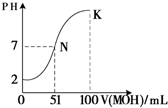
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©¢ŁHCl ¢ŚNaOH ¢ŪCH3COOH ¢ÜNH3”¤H2O ¢ŻCH3COONa ¢ŽNH4ClÖŠ£¬ CH3COOH ”¢NH3”¤H2OŹōÓŚČõµē½āÖŹ£»NaOH”¢NH3”¤H2O”¢CH3COONa ČÜŅŗĻŌ¼īŠŌ£»£Ø2£©³£ĪĀĻĀ0.01 mol/L HClČÜŅŗµÄPH=-lg0.01=2£¬PH=11µÄCH3COONaČÜŅŗÖŠµÄc£ØH+£©=10-11mol/L£¬CH3COONaĪŖĒæ¼īČõĖįŃĪ£¬ĖłŅŌÓÉĖ®µēĄė²śÉśµÄc(OH£) =Kw/c£ØH+£©=10-3 mol£ÆL £»£Ø3£©CH3COONaĪŖĒæ¼īČõĖįŃĪ£¬ÓÉÓŚ“×ĖįøłµÄĖ®½āŹ¹µĆĘäČÜŅŗĻŌ¼īŠŌ£¬ĘäĻŌ¼īŠŌµÄĄė×Ó·½³ĢŹ½ĪŖCH3COO? + H2O  CH3COOH + OH?£¬øł¾ŻµēŗÉŹŲŗćæÉŅŌµĆµ½c£ØH+£©+c£ØNa+£©=c£ØOH?£©+c£ØCH3COO?£©£¬ŅņĪŖČÜŅŗĻŌ¼īŠŌ£¬ĖłŅŌc£ØOH-£©£¾c£ØH+£©£¬¹ŹČÜŅŗÖŠĄė×ÓÅØ¶Č°“Óɓ󵽊”µÄĖ³ŠņĪŖc£ØNa+£©£¾c£ØCH3COO?£©£¾c£ØOH-£©£¾c£ØH+£©£»£Ø4£©HClĪŖĒæĖį£¬¶ų“×ĖįĪŖČõĖį£¬ĖłŅŌ½«µČPHµČĢå»żµÄHClŗĶCH3COOH·Ö±šĻ”ŹĶm±¶ŗĶn±¶£¬Ļ”ŹĶŗóĮ½ČÜŅŗµÄPHČŌĻąµČ£¬ŌņmŠ”ÓŚn£»£Ø5£©ÓÉĶ¼æÉŅŌÖŖµĄ£¬ 0.01 mol”¤L£1HAČÜŅŗpHĪŖ2£¬ĖłŅŌHAĪŖĒæĖį£»¢Ś Kµć¶ŌÓ¦µÄČÜŅŗÖŠ£¬øł¾ŻĪļĮĻŹŲŗćæÉŅŌÖŖµĄ¼ÓČėµÄMOHµÄĪļÖŹµÄĮæĪŖ0.02”Į0.1=0.002mol£¬ĖłŅŌc(M£«)£«c(MOH)=0.002/0.2=0.01mol”¤L£1”£
CH3COOH + OH?£¬øł¾ŻµēŗÉŹŲŗćæÉŅŌµĆµ½c£ØH+£©+c£ØNa+£©=c£ØOH?£©+c£ØCH3COO?£©£¬ŅņĪŖČÜŅŗĻŌ¼īŠŌ£¬ĖłŅŌc£ØOH-£©£¾c£ØH+£©£¬¹ŹČÜŅŗÖŠĄė×ÓÅØ¶Č°“Óɓ󵽊”µÄĖ³ŠņĪŖc£ØNa+£©£¾c£ØCH3COO?£©£¾c£ØOH-£©£¾c£ØH+£©£»£Ø4£©HClĪŖĒæĖį£¬¶ų“×ĖįĪŖČõĖį£¬ĖłŅŌ½«µČPHµČĢå»żµÄHClŗĶCH3COOH·Ö±šĻ”ŹĶm±¶ŗĶn±¶£¬Ļ”ŹĶŗóĮ½ČÜŅŗµÄPHČŌĻąµČ£¬ŌņmŠ”ÓŚn£»£Ø5£©ÓÉĶ¼æÉŅŌÖŖµĄ£¬ 0.01 mol”¤L£1HAČÜŅŗpHĪŖ2£¬ĖłŅŌHAĪŖĒæĖį£»¢Ś Kµć¶ŌÓ¦µÄČÜŅŗÖŠ£¬øł¾ŻĪļĮĻŹŲŗćæÉŅŌÖŖµĄ¼ÓČėµÄMOHµÄĪļÖŹµÄĮæĪŖ0.02”Į0.1=0.002mol£¬ĖłŅŌc(M£«)£«c(MOH)=0.002/0.2=0.01mol”¤L£1”£
æ¼µć£ŗĒæČõµē½āÖŹ”¢ŃĪĄąĖ®½ā”¢µēŗÉŹŲŗć£¬ĪļĮĻŹŲŗć
µćĘĄ£ŗ±¾Ģāæ¼²éĮĖĒæČõµē½āÖŹ”¢ŃĪĄąĖ®½ā”¢µēŗÉŹŲŗć£¬ĪļĮĻŹŲŗćµÄĻą¹ŲÖŖŹ¶£¬ÓŠŅ»¶ØµÄ×ŪŗĻŠŌ£¬±¾ĢāÄѶȏŹÖŠ”£


 Źī¼Ł×÷Ņµŗ£Ńą³ö°ęÉēĻµĮŠ“š°ø
Źī¼Ł×÷Ņµŗ£Ńą³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĻĀĮŠ»ÆŗĻĪļ£ŗ¢ŁHCl ¢ŚNaOH ¢ŪCH3COOH ¢ÜNH3?H2O ¢ŻCH3COONa ¢ŽNH4Cl
ĻĀĮŠ»ÆŗĻĪļ£ŗ¢ŁHCl ¢ŚNaOH ¢ŪCH3COOH ¢ÜNH3?H2O ¢ŻCH3COONa ¢ŽNH4Cl²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģɽ¶«Ź”Ķžŗ£ŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ĻĀĮŠ»ÆŗĻĪļ£ŗ¢ŁHCl ¢ŚNaOH ¢ŪCH3COOH ¢ÜNH3”¤H2O ¢ŻCH3COONa ¢ŽNH4Cl
£Ø1£©ŹōÓŚČõµē½āÖŹµÄŹĒ £¬ČÜŅŗ³Ź¼īŠŌµÄÓŠ £ØĢīŠņŗÅ£©”£
£Ø2£©³£ĪĀĻĀ0.01 mol/L HClČÜŅŗµÄPH= £»PH=11µÄCH3COONaČÜŅŗÖŠÓÉĖ®µēĄė²śÉśµÄc(OH£) = ”£
£Ø3£©ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾CH3COONaČÜŅŗ³Ź¼īŠŌµÄŌŅņ £¬ĘäČÜŅŗÖŠĄė×ÓÅØ¶Č°“Óɓ󵽊”µÄĖ³ŠņĪŖ ”£
£Ø4£©½«µČPHµČĢå»żµÄHClŗĶCH3COOH·Ö±šĻ”ŹĶm±¶ŗĶn±¶£¬Ļ”ŹĶŗóĮ½ČÜŅŗµÄPHČŌĻąµČ£¬Ōņm n £ØĢī”°“óÓŚ”¢µČÓŚ”¢Š”ÓŚ”±£©”£
£Ø5£©³£ĪĀĻĀ£¬Ļņ100 mL 0.01 mol”¤L£1HAČÜŅŗÖšµĪ¼ÓČė0.02 mol”¤L£1MOHČÜŅŗ£¬Ķ¼ÖŠĖłŹ¾ĒśĻß±ķŹ¾»ģŗĻČÜŅŗµÄpH±ä»ÆĒéæö£ØĢå»ż±ä»ÆŗöĀŌ²»¼Ę£©”£»Ų“šĻĀĮŠĪŹĢā£ŗ

¢ŁÓÉĶ¼ÖŠŠÅĻ¢æÉÖŖHAĪŖ_______Ėį£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
¢Ś Kµć¶ŌÓ¦µÄČÜŅŗÖŠ£¬
c(M£«)£«c(MOH)= mol”¤L£1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½¶«Ź”Ķžŗ£ŹŠø߶ž£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com