
Ϊ�ⶨ̼���Ƶ������������躬����̼�����ƣ���ijѧ���������������ʵ�鷽������ش�ÿ�������е����⡣
 [������] ��������
[������] ��������
| |||
|
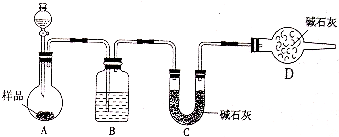
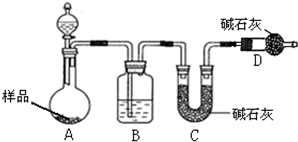
��1����������Ҫ�������� ��
��2����Ʒ���� ���������ա�
a.������ b.�ձ� c.����
��3��ʵ���в���A������Ϊ ��
��4��������պ����Ʒ���ڿ�������ȴ�������ʵ���� ���ƫ����ƫС�����䡱����
��5����Ʒ̼���Ƶ��������� ����֪����Ʒ̼���Ƶ���������Ϊ0.913����ʵ���������Ϊ ��
[����II] �ζ������ٳ�ȡ��Ʒ M g������amol/L ��������VmL���ձ����ܽ���Ʒ������ˮϡ�����100mL��Һ����ȡ�ܽ�����Һ20.00mL����bmol/L NaOH��Һ�ζ���ǡ����ȥV��mL�����ظ��۵IJ���2~3�Ρ�
��6�����������Һ����Ҫ�������������� ���ζ��л���Ҫ���Լ��� ��
��7�������ܵ�Ŀ���� ��
��8���ζ���õĹ��������к�HCl�������ʵ����ı���ʽΪ ��
��9�����в�����ʹ���������к�HCl�ⶨֵƫС���� ��
a.ʢ���������Ƶĵζ���ֻ������ˮϴ��
b.�ζ���������Һ�彦��
c.�ζ���������ˮ��ϴ��ƿ�ڱ�
��10����������������Ϊ2�������У��Ϻõķ����� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
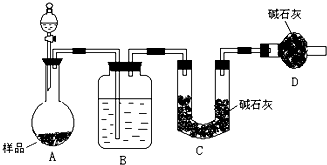 ijУ��ѧ�о�ѧϰС���������ʵ�鷽�����ⶨС�մ���Ʒ�д��������������
ijУ��ѧ�о�ѧϰС���������ʵ�鷽�����ⶨС�մ���Ʒ�д���������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
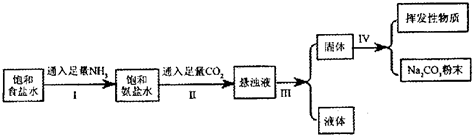
| ���� | NH4Cl | NaHCO3 | Na2CO3 | NaCl |
| �ܽ��/g | 37.2 | 9.6 | 21.5 | 36.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ�ⶨ̼������̼�����ƻ������Ʒ��̼���Ƶ������������������ʵ�鷽����
Ϊ�ⶨ̼������̼�����ƻ������Ʒ��̼���Ƶ������������������ʵ�鷽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ��ⶨ��Ʒ�гɷֺ�����ʵ��Ӧ�ظ��������Ρ�Ϊ�˲ⶨij�������ƹ����л��е�̼���Ƶ������������ס�����λͬѧ�ֱ����������ʵ�鷽����
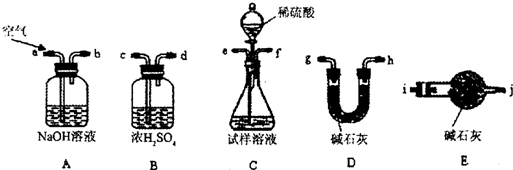
I����ͬѧ�ķ�����ͼ��ʾ��
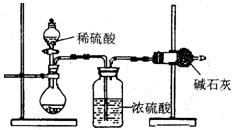
(1)���ݼ�ͬѧ��ʵ��װ��ͼ��������ÿ��ʵ������������еij�����������Ҫ���� �Ρ�
(2)��ͬѧ�ظ��ⶨ�����Σ��õ�̼���Ƶ�������
�������ݴ��ڽϴ��ƫ�����Ϊԭ������� (�����)��
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ������
B��װ��������е�ˮ�����Ͷ�����̼����ʯ������
C����Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ������
D������ϡ����������㣬�����������
II����ͬѧ�ķ����ǣ���ȡ��Ʒm g�����ܽ⣬�ӹ����Ȼ�����Һ�����ˡ�ϴ�ӡ���ɣ�
�����ù���n g��
(1)�������̼���Ƶ���������Ϊ(��m��n��ʾ) ��
(2)ϴ�ӳ����IJ���Ҫ���� ��
(3)Ca2+��Ba2+������ʹCO32-������ȫ������ͬѧʹ���Ȼ�����Һ�������Ȼ�����Һ��ԭ���� ���ⶨCO32-��������ʹ������������Һ����������������Һ����������и��ߵľ�ȷ�ȣ�ԭ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com