
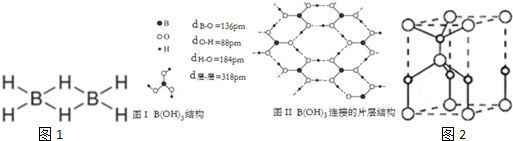
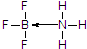 £»
£»·ÖĪö £Ø1£©øł¾ŻŌŖĖŲĆū³Ę£¬ÅŠ¶ĻŌŖĖŲŌ×ÓµÄŗĖĶāµē×ÓŹż£¬ŌŁøł¾ŻŗĖĶāµē×ÓÅŲ¼¹ęĀÉĄ“Š“£¬ÅšŌŖĖŲĪŖ5ŗÅŌŖĖŲ£»øł¾Ż¼Ū²ćµē×Ó¶Ō»„³āĄķĀŪČ·¶ØĘäŌÓ»ÆĄąŠĶ£»
£Ø2£©øł¾Ż¾§ĢåµÄČŪ·ŠµćæÉÅŠ¶Ļ¾§ĢåĄąŠĶ£¬ÅšŌŖĖŲ¾ßӊȱµē×ÓŠŌ£¬Ęä»ÆŗĻĪļæÉÓė¾ßÓŠ¹Āµē×Ó¶ŌµÄ·Ö×Ó»ņĄė×ÓŠĪ³ÉÅäŗĻĪļ£¬BF3ÄÜÓėNH3·“Ӧɜ³ÉBF3•NH3£¬NŌ×Óŗ¬ÓŠ¹Āµē×Ó¶Ō£»
£Ø3£©¢ŁÅšĖįĪŖŅ»ŌŖČõĖį£¬ŌŚĖ®ČÜŅŗĄļŗĶĖ®½įŗĻŠĪ³ÉÅäĪ»¼ü£¬²æ·ÖµēĄė³öŅõŃōĄė×Ó£»
¢Śa£®øł¾ŻÅšĖį¾§ĢåĪŖʬ²ćד½į¹¹·ÖĪö£»
b£®·Ö×ÓµÄĪČ¶ØŠŌÓė»Æѧ¼üÓŠ¹Ų£»
c£®ĄūÓĆ¾łĢƷؼĘĖćŗ¬1molH3BO3µÄ¾§ĢåÖŠµÄĒā¼ü£¬ŗ¬1mol H3BO3µÄ¾§ĢåÖŠÓŠ3molĒā¼ü£»
d£®Óɽį¹¹æÉÖŖ£¬ÅšŌ×Ó×īĶā²ćÖ»ÓŠ3øöµē×Ó£¬ÓėŃõŌ×ÓŠĪ³É3¶Ō¹²ÓƵē×Ó¶Ō£»
£Ø4£©¢ŁĄūÓĆ¾łĢƷؼĘĖćŌ×ÓøöŹż£¬Į½ÖÖŌŖĖŲŌ×ÓøöŹż×ī¼ņ±ČČ·¶Ø»ÆѧŹ½£»
¢ŚĶ¬Ņ»ÖÜĘŚŌŖĖŲÖŠ£¬ŌŖĖŲµÄµŚŅ»µēĄėÄÜĖę×ÅŌ×ÓŠņŹżŌö“ó¶ų³ŹŌö“óĒ÷ŹĘ£¬µ«µŚIIA×唢µŚVA×åŌŖĖŲµŚŅ»µēĄėÄÜ“óÓŚĘäĻąĮŚŌŖĖŲ£®
½ā“š ½ā£ŗ£Ø1£©BŌŖĖŲĪŖ5ŗÅŌŖĖŲ£¬Ō×ÓŗĖĶāÓŠ5øöµē×Ó£¬·ÖĮ½²ćÅŲ¼£¬µŚŅ»²ć2øö£¬µŚ¶ž²ć3øö£¬ĖłŅŌŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ£ŗ1s22s22p1£¬ŅŅÅšĶé·Ö×ÓÖŠĆæøöÅšŌ×Óŗ¬ÓŠ4øö¹²¼Ū¼ü£¬ĖłŅŌBŌ×Ó²ÉÓĆsp3Ōӻƣ¬
¹Ź“š°øĪŖ£ŗ1s22s22p1£»sp3Ōӻƣ»
£Ø3£©ČżĀČ»ÆÅšŗĶČż·ś»ÆÅš³£ĪĀĻĀ¶¼ŹĒĘųĢ壬ĖłŅŌĖüĆĒ¹ĢĢ¬Ź±µÄ¾§ĢåĄąŠĶĪŖ·Ö×Ó¾§Ģ壬ŚŌŖĖŲ¾ßӊȱµē×ÓŠŌ£¬Ęä»ÆŗĻĪļæÉÓė¾ßÓŠ¹Āµē×Ó¶ŌµÄ·Ö×Ó»ņĄė×ÓŠĪ³ÉÅäŗĻĪļ£¬BF3ÄÜÓėNH3·“Ӧɜ³ÉBF3•NH3£¬BÓėNÖ®¼äŠĪ³ÉÅäĪ»¼ü£¬NŌ×Óŗ¬ÓŠ¹Āµē×Ó¶Ō£¬ĖłŅŌµŖŌ×ÓĢį¹©¹Āµē×Ó¶Ō£¬BF3•NH3½į¹¹Ź½ĪŖ£ŗ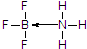 £¬
£¬
¹Ź“š°øĪŖ£ŗ·Ö×Ó¾§Ģ壻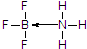 £»
£»
£Ø3£©¢ŁÅšĖįĪŖŅ»ŌŖČõĖį£¬ŌŚĖ®ČÜŅŗĄļ£¬ŗĶĖ®µēĄė³öµÄĒāŃõøłĄė×ÓŠĪ³ÉÅäĪ»¼ü£¬ĘäµēĄė·½³ĢŹ½ĪŖ£ŗH3BO3+H2O?[B£ØOH£©4]-+H+£¬
¹Ź“š°øĪŖ£ŗH3BO3+H2O?[B£ØOH£©4]-+H+£»
¢Śa£®ÅšĖį£ØH3BO3£©ŹĒŅ»ÖÖʬ²ćד½į¹¹°×É«¾§Ģå£¬Ę¬²ćד½į¹¹¾§ĢåÓŠ»¬ÄåøŠ£¬æÉ×÷Č󻬼Į£¬¹ŹaÕżČ·£»
b£®·Ö×ÓµÄĪČ¶ØŠŌÓė·Ö×ÓÄŚµÄB-O”¢H-O¹²¼Ū¼üÓŠ¹Ų£¬ČŪ·ŠµćÓėĒā¼üÓŠ¹Ų£¬¹Źb“ķĪó£»
c£®1øöÅšĖį·Ö×ÓŠĪ³ÉĮĖ6øöĒā¼ü£¬µ«ĆæøöĒā¼üŹĒ2øöÅšĖį·Ö×Ó¹²ÓĆµÄ£¬ĖłŅŌĘ½¾łŗ¬3øöĒā¼ü£¬Ōņŗ¬ÓŠ1molH3BO3µÄ¾§ĢåÖŠÓŠ3molĒā¼ü£¬¹ŹcÕżČ·£»
d£®ÅšŌ×Ó×īĶā²ćÖ»ÓŠ3øöµē×Ó£¬ÓėŃõŌ×ÓŠĪ³É3¶Ō¹²ÓƵē×Ó¶Ō£¬Ņņ“ĖBŌ×Ó²»ŹĒ8e-ĪČ¶Ø½į¹¹£¬¹Źd“ķĪó£»
¹Ź“š°øĪŖ£ŗac£»
£Ø4£©¢Łøł¾ŻŌ×Ó°ė¾¶“óŠ”ÖŖ£¬BŌ×Ó°ė¾¶“óÓŚNŌ×Ó£¬ĖłŅŌ°×É«Ēņ±ķŹ¾BŌ×Ó£¬ĄūÓĆ¾łĢƷصĆBŌ×ÓøöŹż=1+8”Į$\frac{1}{8}$=2£¬NŌ×ÓøöŹż=1+4”Į$\frac{1}{4}$=2£¬BŌ×ÓŗĶNŌ×ÓøöŹżÖ®±ČĪŖ2£ŗ2=1£ŗ1£¬ĖłŅŌĘä»ÆѧŹ½ĪŖBN£¬
¹Ź“š°øĪŖ£ŗBN£»
¢ŚĶ¬Ņ»ÖÜĘŚŌŖĖŲÖŠ£¬ŌŖĖŲµÄµŚŅ»µēĄėÄÜĖę×ÅŌ×ÓŠņŹżŌö“ó¶ų³ŹŌö“óĒ÷ŹĘ£¬µ«µŚIIA×唢µŚVA×åŌŖĖŲµŚŅ»µēĄėÄÜ“óÓŚĘäĻąĮŚŌŖĖŲ£¬ĖłŅŌµŚŅ»µēĄėÄܽéÓŚB”¢NÖ®¼äµÄµŚ¶žÖÜĘŚŌŖĖŲÓŠBe”¢C”¢O£¬ĖłŅŌÓŠ3ÖÖ£¬
¹Ź“š°øĪŖ£ŗ3£®
µćĘĄ ±¾Ģāæ¼²éĮĖÓŠ¹ŲÅšµÄÖŖŹ¶£¬²ąÖŲæ¼²éŌ×ÓŗĖĶāµē×ÓÅŲ¼”¢ŌÓ»ÆĄķĀŪµÄÓ¦ÓĆ”¢ÅšĖį¾§Ģ徧ĢåĄąŠĶµÄÅŠ¶Ļ”¢Ó°Ļģ·Ö×ÓĪČ¶ØŠŌµÄŅņĖŲµČÖŖŹ¶µć£¬ĢāÄæÄѶČÖŠµČ£¬×¢Ņā·Ö×ÓµÄĪČ¶ØŠŌÓė»Æѧ¼üÓŠ¹Ų£¬ĪļÖŹµÄČŪ·ŠµćÓėĒā¼üÓŠ¹Ų£¬×¢ŅāÅšĖį£ØH3BO3£©ŹĒŅ»ÖÖʬ²ćד½į¹¹°×É«¾§Ģ壮



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
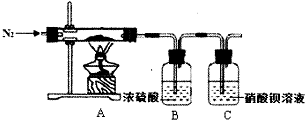 ĮņĖįŃĒĢśļ§ÓÖ³ĘĪŖĦ¶ūŃĪ£¬ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬Ęä×é³ÉæɱķŹ¾ĪŖx£ØNH4£©2S04•yFeS04•zH20£®Ä³ŠĖȤŠ”×éĢ½¾æ×é³ÉÖŠµÄx”¢y”¢zµÄŹżÖµ£®
ĮņĖįŃĒĢśļ§ÓÖ³ĘĪŖĦ¶ūŃĪ£¬ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬Ęä×é³ÉæɱķŹ¾ĪŖx£ØNH4£©2S04•yFeS04•zH20£®Ä³ŠĖȤŠ”×éĢ½¾æ×é³ÉÖŠµÄx”¢y”¢zµÄŹżÖµ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬1 mol CCl4µÄĢå»żŌ¼ĪŖ22.4L | |
| B£® | NaOHµÄĦ¶ūÖŹĮæŹĒ40g | |
| C£® | ³£ĪĀ³£Ń¹ĻĀ£¬11.2LŃõĘųÓė×ćĮæĶ·Ū³ä·Ö·“Ó¦£¬×ŖŅʵĵē×ÓŹżĪŖ2NA | |
| D£® | 46g NO2 ŗĶ46g N2O4 ŗ¬ÓŠµÄŌ×ÓŹż¾łĪŖ3NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CĻūŗĵÄĖŁĀŹÓėAĻūŗĵÄĖŁĀŹĻąµČ | |
| B£® | µ„Ī»Ź±¼äÄŚÉś³Éa mol B£¬Ķ¬Ź±ĻūŗÄa mol C | |
| C£® | ČŻĘ÷ÄŚµÄŃ¹Ēæ²»ŌŁ±ä»Æ | |
| D£® | »ģŗĻĘųĢåµÄĆÜ¶Č²»ŌŁ±ä»Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | KHCO3ŗĶMgCO3 | B£® | K2CO3ŗĶNa2CO3 | C£® | MgCO3ŗĶNa2CO3 | D£® | KHCO3ŗĶNaHCO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
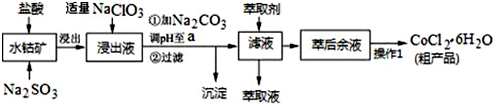
| ³ĮµķĪļ | Fe£ØOH£©3 | Fe£ØOH£©2 | Co£ØOH£©2 | Al£ØOH£©3 | Mn£ØOH£©2 |
| æŖŹ¼³Įµķ | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ĶźČ«³Įµķ | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
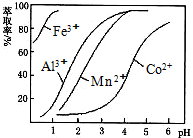
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com