
����0.175mol/L��������Һ500mL(��֪����ĵ��볣��Ka=1.75x10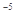 )
)
��1��д��������ˮ�ⷴӦ�Ļ�ѧ����ʽ_____________________��
��2������ͼ����˵�������Ƶ�ˮ�ⷴӦ�ﵽƽ�����_____________________��
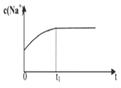 | 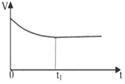 | 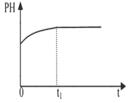 |  |
| A����Һ��c (Na��)�뷴Ӧʱ��t�Ĺ�ϵ | B.CH3COO����ˮ�������뷴Ӧʱ��t�Ĺ�ϵ | C.��Һ��PH�뷴Ӧʱ��t�Ĺ�ϵ | D.KW�뷴Ӧʱ��t�Ĺ�ϵ |
��1��CH3COONa +H2O CH3COOH+NaOH
CH3COOH+NaOH
��2��BC(ѡ1����1�֣�����������)
��3��CD
��4��AC
��5��7.2(��7.175�ĸ�1��)��0.35mol/L����λ��1�֣�
��6��9
���������������1��������ˮ�����ɴ�����������ƣ���ѧ����ʽΪCH3COONa +H2O CH3COOH+NaOH
CH3COOH+NaOH
��2��A�������Ӳ�ˮ�⣬����Ũ��ʼ�ղ��䣬����B����������ӿ�ʼʱˮ�����������С��ƽ��ʱ���ڱ仯����ȷ��C������ˮ������У�pH������ƽ��ʱ���ڱ仯����ȷ��D��KW��һ�¶ȳ������¶Ȳ��䣬KW���䣬����ѡBC��
��3��A�������������Һ�д���Ũ������ƽ�����ƣ�����B�����봿����壬��ƽ����ϵ������Ũ����Ӱ�죬ƽ�ⲻ�ƶ�������C���������ƹ��壬��Һ�ڴ��������Ũ������ƽ�����ƣ���ȷ��D�������Ȼ�粒��壬笠�������ˮ�����ɵ����������ӽ�ϳ�һˮ�ϰ���ʹ��Һ������������Ũ�ȼ�С��ƽ�����ƣ���ȷ����ѡCD��
��4��A����������ᣬʹ���������Ũ������������Ũ�Ȳ��䣬����A��ȷ��B���������������ᣬƽ�����ƣ����������Ũ��������������Ũ�ȣ�����C����������ᣬ����Һ�д���Ũ�Ƚϴ�ʱ������ĵ�����ڴ�������ӵ�ˮ��̶ȣ����������Ũ��������Һ�����ԣ���ȷ��D�������Ƿ����̶ȴ���ˮ��̶ȣ����������c(OH-)��c(Na+),����ѡAC��
��5����m=nM�ô����Ƶ�����Ϊ7.175g������������ƽ����������Ϊ7.2g���������������Ƶ�Ũ�ȵ������ϣ���Ϻ����ҺŨ�ȼ���Ϊ0.175mol/L������ԭ����Ũ��Ϊ0.35mol/L
��6�� �������ˮ�ⷴӦ��ƽ�ⳣ��
K=Kw��Ka(CH3COOH)=c��CH3COOH��c(OH-)/c(CH3COO-)= c(OH-)2/c(CH3COO-),����c(OH-)=10-5��Ph=9
���㣺�����ε�ˮ�⣬��Һ����������ԭ����Ӧ�ã�����Ũ�ȵıȽϣ�pH�ļ���


 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ˮ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ��ͳ������ܽ�ƽ�⡣�������ѧ֪ʶ�ش��������⣺
(1)NaHCO3��Һ�й�����7������������Na����HCO ��H����CO
��H����CO ��H2O��________��________(�������)��
��H2O��________��________(�������)��
(2)�����£����ʵ���Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ��NH4NO3����NaCl����Na2CO3����H2SO4����NaOH����CH3COONa��pH�Ӵ�С����˳��Ϊ____________��
(3)����ʱ��AlCl3��ˮ��Һ��________(��ᡱ�����С������)�ԣ�ԭ����(�����ӷ���ʽ��ʾ)��_______________________________________
ʵ����������AlCl3����Һʱ��Ϊ������AlCl3��ˮ��ɼ���������________(��д���ʵ�����)����AlCl3��Һ���ɣ����գ����õ�����Ҫ���������________��
(4)�����£����ʵ���Ũ����ͬ��������Һ����NH4Cl����(NH4)2SO4����NH3��H2O����(NH4)2CO3����NH4HSO4����Һ��c(NH )�Ӵ�С��˳��Ϊ��____________(�����)��
)�Ӵ�С��˳��Ϊ��____________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ���¶���,�������ˮϡ������,��Һ�ĵ�����������ͼ��ʾ,��ش�:
(1)��O���㵼������Ϊ0��������������������������
(2)A��B��C������Һc(H+)��С�����˳��Ϊ��������������������������������������
(3)��ʹC����Һ��c(CH3COO-)�����ͬʱ��Һ��c(H+)��С,�ɲ�ȡ�Ĵ�ʩ��:
������������;������������;��������������
(4)��ʵ����C�㴦:c(CH3COOH)="0.1" mol��L-1,c(CH3COO-)="0.01" mol��L-1,���������CH3COOH�ĵ��볣��Ka=����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(����Ԥ����)��֪25 ��ʱ������ʵĵ���ƽ�ⳣ����
Ka(CH3COOH)��1.8��10��5��Ka(HSCN)��0.13��
(1)��20 mL 0.10 mol��L��1 CH3COOH��Һ��20 mL 0.10 mol��L��1��HSCN��Һ�ֱ���0.10 mol��L��1��NaHCO3��Һ��Ӧ��ʵ���ò���CO2�������(V)��
ʱ��t�Ĺ�ϵ��ͼ��ʾ��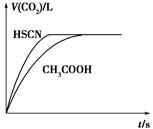
��Ӧ��ʼʱ��������Һ����CO2���������Բ�ͬ��ԭ����________����Ӧ������������Һ��c(SCN��)______c(CH3COO��)(���������������)��
(2)2.0��10��3 mol��L��1�������ˮ��Һ�У�������ҺpH(���Ե���ʱ����仯)�����ƽ����ϵ��c(F��)��c(HF)����ҺpH�Ĺ�ϵ��ͼ��ʾ����25 ��ʱ��HF����ƽ�ⳣ��ΪKa(HF)��________(��ʽ��ֵ)��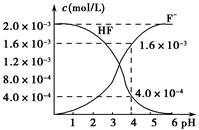
(3)��������CaF2�ܶȻ�����ΪKsp��1.5��10��10����4.0��10��3 mol��L��1 HF��Һ��4.0��10��4 mol��L��1��CaCl2��Һ�������ϣ�������ҺpH��4(���Ե���ʱ��Һ����仯)���Է�����Ϻ��Ƿ��г������ɣ�________(��С���û�С�)���������ɣ�_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ﮱ���Ϊ������ζ��������LiCoO2Ϊ�������ϵ�����ӵ���ѱ��㷺������Яʽ��Դ����ҵ�ϳ��Ԧ�-﮻Կ�(��Ҫ�ɷ�ΪLiAlSi2O6��������FeO��MgO��CaO������)Ϊԭ������ȡ����ﮡ�����һ�ֹ����������£�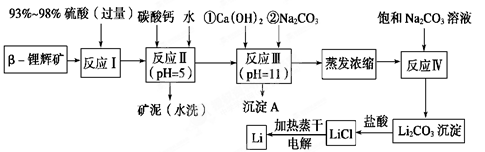
��֪���ٲ��ֽ����������↑ʼ��������ȫ����ʱ��pH��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 2��7 | 3.7 | 9.6 |
| ��ȫ����pH | 3.7 | 4.7 | 11 |
| �¶�/�� | 0 | 10 | 20 | 50 | 75 | 100 |
| Li2CO3���ܽ��/g | 1.539 | 1.406 | 1.329 | 1.181 | 0.866 | 0.728 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I�����ú��̷�ˮ����Ҫ��Mn2+��SO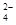 ��H+��Fe2+��Al3+��Cu2+�����Ʊ������ܴ��Բ���̼���̣�MnCO3��������һ�ֹ�ҵ�������£�
��H+��Fe2+��Al3+��Cu2+�����Ʊ������ܴ��Բ���̼���̣�MnCO3��������һ�ֹ�ҵ�������£�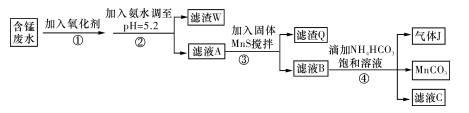
��֪ijЩ������ȫ������pH���±���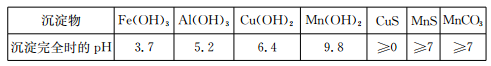
�ش��������⣺
��1�����̢��У�������������Ҫ�ɷ��� ��
��2�����̢��У�������Ӧ�����ӷ���ʽ�� ��
��3�����̢��У������ɵ�����J��ʹ����ʯ��ˮ����ǣ�������MnCO3�ķ�Ӧ�����ӷ���ʽ�� ��
��4����MnCO3���Ƶ���Ҫ�Ĵ���MnO2��2MnCO3+O2=2MnO2+2CO2��
���ڿ����м���460��0 g MnCO3���õ�332��0 g��Ʒ������Ʒ������ֻ��MnO����ò�Ʒ��MnO2������������ ���ðٷ�����ʾ��С�������1λ����
�����£�Ũ�Ⱦ�Ϊ0��1 mol��L������������Һ��pH���±���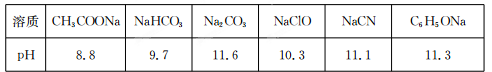
��1����������Һ�е������ӣ����H+������ǿ���� ��
��2�����ݱ��������жϣ�Ũ�Ⱦ�Ϊ0��0l mol��L���������ʵ���Һ�У�������ǿ����
������ţ���
A��HCN B��HC1O C��C6H5OH D��CH3 COOH E��H2 CO3
��3�����ϱ����ݣ������ж����з�Ӧ���ܳ������� ������ţ���
A��HCN+ Na2 CO3=NaHCO3+NaCN
B��CH3 COOH+NaCN=CH3 COONa+HCN
C��CO2 +H2O+2C6 H5ONa=Na2 CO3 +2C6 H5OH
D��CH3 COONa+HClO=NaClOʮCH3 COOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ũ�Ⱦ�Ϊ0��1mol ��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ�李��ݴ���李���������李��߰�ˮ����ش��������⣺
��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ�李��ݴ���李���������李��߰�ˮ����ش��������⣺
(l)�١��ڡ��ۡ���������Һ����ˮ�������Ũ���ɴ�С��˳����___________________
_____________________________________������ţ���
��2���ܡ��ݡ��ޡ���������Һ��NH4+Ũ���ɴ�С��˳����_________________������ţ���
��3�����ۺܰ͢������l��2��Ϻ���Һ��pH>7������Һ�и�����Ũ���ɴ�С��˳����________________________________________��
��4����֪t��ʱ��Kw=1��10 ����t��__________���>������<����=����25�档��t��ʱ��pH=11��NaOH��ҺaL��pH=1��H2SO4��ҺbL��ϣ����Ի�Ϻ���Һ����ı仯���������û����Һ��pH=2���� a:b=______________��
����t��__________���>������<����=����25�档��t��ʱ��pH=11��NaOH��ҺaL��pH=1��H2SO4��ҺbL��ϣ����Ի�Ϻ���Һ����ı仯���������û����Һ��pH=2���� a:b=______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��0.10 mol��L-1����ͭ��Һ�м�����������ϡ��Һ��ֽ�����dz��ɫ������ͭ�������ɣ�����Һ��pH=8ʱ��c(Cu2+)=
mol��L-1(Ksp��Cu(OH)2��=2.2��10-20)��
(2)����0.1 mol��L-1����ͭ��Һ��ͨ�����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�H+Ũ���� mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A��B��C��D��E���ֿ���ǿ����ʣ�������ˮ�пɵ�������������ӣ��������Ӳ��ظ�����
| ������ | H+��Na+��Al3+��Ag+��Ba2+ |
| ������ | OH-��Cl-��CO32-��NO3-��SO42- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com