
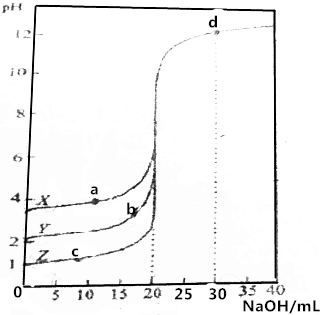
 ��0.1000mol•L-1��NaOH��Һ�ֱ�ζ�0.1000mol•L-1��20.00mLX��Y��Z��������Һ����ҺpH�����NaOH���֮��Ĺ�ϵ��ͼ��ʾ������˵��������ǣ�������
��0.1000mol•L-1��NaOH��Һ�ֱ�ζ�0.1000mol•L-1��20.00mLX��Y��Z��������Һ����ҺpH�����NaOH���֮��Ĺ�ϵ��ͼ��ʾ������˵��������ǣ�������| A�� | ZΪһԪǿ�� | |
| B�� | d���c��OH-��Ϊ0.02000mol•L-1 | |
| C�� | a��b��c��b��������ӵ����ʵ���Ũ����� | |
| D�� | X��YΪһԪ���ᣬ������볣����Ka��x����Ka��Y�� |
���� A������Һ����ȫ�������Ϊǿ�
B��d����Һ��pH=12������c��OH-��=$\frac{Kw}{c��{H}^{+}��}$���㣻
C��d���Ǽ����NaOH����������������Ũ�����
D����Ũ�ȵ�һԪ��������������Ũ��Խ����̶�Խ��
��� �⣺A������Һ����ȫ�������Ϊǿ�ᣬ0.1000mol•L-1Z��Һ��pH=1��˵��Z��ȫ���룬��ZΪǿ�ᣬ��A��ȷ��
B��d����Һ��pH=12����c��H+��=10-12mol/L������c��OH-��=$\frac{Kw}{c��{H}^{+}��}$=0.01mol/L����B����
C��d���Ǽ����NaOH����������������Ũ������������ɵ��ε�Ũ������ε�����������ӵ����ʵ���Ũ�����C��ȷ��
D����Ũ�ȵ�һԪ��������������Ũ��Խ����̶�Խ��Ũ�Ⱦ�Ϊ0.1000mol•L-1ʱY��pHС����Y��������Ũ�ȴ�����Y�ĵ��볣��������볣����Ka��x����Ka��Y������D��ȷ��
��ѡB��
���� ���⿼��������к͵ζ����ߣ�����ͼ�����߱仯ȷ�����ǿ������Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ��������ͼ���������


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ʒ���Ƴ���ҺV1L��ȡ����25.00mLǡ����V2mLŨ��Ϊcmol/L����KMnO4��Һ��ȫ��Ӧ | |
| B�� | ����Ʒ�м�����H2O2���ټ�����BaCl2��Һ�����ˣ�������ϴ�ӡ��������������Ϊbg | |
| C�� | ����Ʒ������ϡ�����ַ�Ӧ���ټ�������BaCl2��Һ�����ˣ�������ϴ�ӡ��������������Ϊc g | |
| D�� | ����Ʒ������ϡ�����ַ�Ӧ�����ɵ���������ͨ��ʢ�б���NaHSO3��ϴ��ƿ��ʢ��ŨH2SO4��ϴ��ƿ��ʢ�м�ʯ�ҵĸ���ܢ�ʢ�м�ʯ�ҵĸ���ܢⶨ����ܢ�����d g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
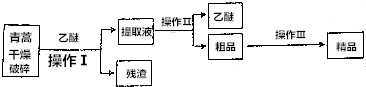
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�0.1mol•L-1��CH3COOH��Һ�У���ˮ�������c��H+��Ϊ1.0��10-13mol•L-1 | |
| B�� | pH=2��pH=1��CH3COOH��Һ��c��H+��֮��Ϊ1��10 | |
| C�� | �����£������pH=12��NaOH��Һ��pH=2��CH3COOH��Һ��ϣ���Ϻ���Һ��pH��7 | |
| D�� | 25��ʱ����ȫ�к�50mLpH=3��H2SO4��Һ����ҪpH=11��NaOH��Һ50mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
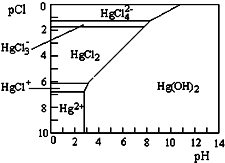 ˮ���ж��۹����ӿ�������������ӽ�ϳɲ�ͬ�Ĵ�����̬��ˮ��Һ�ж��۹���Ҫ������̬��Cl-��OH-��Ũ�ȹ�ϵ��ͼ��ʾ[ע������Ũ�Ⱥ�Сʱ���ø�������ʾ����pH=-lgc��H+����pCl=-lgc��Cl-��]������˵��������ǣ�������
ˮ���ж��۹����ӿ�������������ӽ�ϳɲ�ͬ�Ĵ�����̬��ˮ��Һ�ж��۹���Ҫ������̬��Cl-��OH-��Ũ�ȹ�ϵ��ͼ��ʾ[ע������Ũ�Ⱥ�Сʱ���ø�������ʾ����pH=-lgc��H+����pCl=-lgc��Cl-��]������˵��������ǣ�������| A�� | ������ˮ��Cl-��Ũ�ȴ���0.1mol•L-1���й�Ԫ�ص���Ҫ������̬��HgCl42- | |
| B�� | ����Hg��NO3��2����0.001mol•L-1�������Ԫ�ص���Ҫ������̬��HgCl2 | |
| C�� | ��Hg��NO3��2����ֱ������ˮ��������Һ | |
| D�� | ���÷�ͭм������Hg2+�ķ�ˮ�������ӷ���ʽΪCu+Hg2+�TCu2++Hg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a��b | B�� | a��b | C�� | a=b | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5 | B�� | 8 | C�� | 9 | D�� | 13 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
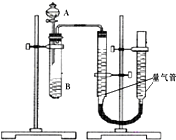 �ס�������С����������KMnO4��H2C2O4��Һ��Ӧ�����ʵ��̽��Ӱ�췴Ӧ���ʵ����أ�2MnO4-+5H2C2O4+6H+=2Mn2++10CO2+8H2O��
�ס�������С����������KMnO4��H2C2O4��Һ��Ӧ�����ʵ��̽��Ӱ�췴Ӧ���ʵ����أ�2MnO4-+5H2C2O4+6H+=2Mn2++10CO2+8H2O��| ��� | A��Һ | B��Һ |
| �� | 2ml 0.2mol/LH2C2O4��Һ | 4ml 0.01mol/LKMnO4��Һ |
| �� | 2ml 0.1mol/LH2C2O4��Һ | 4ml 0.01mol/LKMnO4��Һ |
| �� | 2ml 0.2mol/LH2C2O4��Һ | 4ml 0.01mol/LKMnO4��Һ������MnSO4 |
| ʵ���� | 1 | 2 | 3 | 4 |
| ˮ/ml | 10 | 5 | 0 | X |
| 0.5mol/L H2C2O4/ml | 5 | 10 | 10 | 5 |
| 0.2mol/L KMnO4/ml | 5 | 5 | 10 | 10 |
| ʱ��/s | 40 | 20 | 10 | --- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �������м���ʹKMnO 4������Һ��ɫ��������ˮ��Ӧʹ��ˮ��ɫ���ǣ�������
�������м���ʹKMnO 4������Һ��ɫ��������ˮ��Ӧʹ��ˮ��ɫ���ǣ�������| A�� | �ڢܢݢ� | B�� | �ڢݢ� | C�� | �ڢܢݢ� | D�� | �ڢܢݢߢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com