
��ͼ���й����仯�����ʵ��װ�ã�
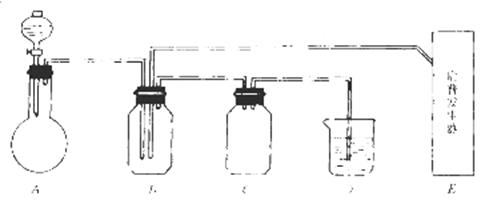
ʵ�����õ���ʵ���Լ���������ѡ��Ũ�����70%�����25%������¿�����������Ʒ�ĩ������������������������Һ������ˮ����̼������Һ��
��ʵ�鿪ʼ���ü���Bƿ�����������ĩ״���ʡ��Իش𣺣��б�ŵı������ţ�
��1��A�з�Һ©��ʢ�ŵ��Լ��� ��
��2��B�з�Ӧ����������ͻ�ԭ��������ʵ������� ��
��3��E�����շ���������ʢ�е������Լ��� ��������Ӧ�����ӷ���ʽ�� ��
��4�����A��E��װ�����巢���ٶ���ͬ������Ҳ��ͬʱ����D�з�Ӧ�����ӷ���ʽ�� ��
��5��D�������� ��C�������� ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ʵ�����õ����Լ����������Լ���ѡ��
����������Ϊ98%��ŨH2SO4 ����������Ϊ70%���������������Ϊ25%��H2SO4 ���¿����Na2SO3��ĩ ������������״���壬������ˮ�� ��NaOH��Һ ����ˮ��Na2CO3��Һ
��ʵ�鿪ʼ�ü���Bƿ�г��ֹ����״���ʡ�������������⣺
��1��A�з�Һ©��ʢ�ŵ��Լ���___________����ƿ��ʢ�ŵ��Լ���___________����ʵ�鿪ʼʱ��Ӧ�Ļ�ѧ����ʽ��_________________________________��
��2��B�з�Ӧ�Ļ�ѧ����ʽ��____________________________________________����Ӧ�����������뻹ԭ��������ʵ���֮����______________________��
��3��E�����շ���������ʢ�ŵ������Լ�������Ӧ�Ļ�ѧ����ʽ��______________________��
��4�����A��E��װ���������ɵ��ٶ���ͬ������Ҳ��ͬ����D�з�����Ӧ�Ļ�ѧ����ʽ��_________________________________��
��5��D��������______________________��C��������_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ʵ�����õ����Լ����������Լ���ѡ��
����������Ϊ98%��ŨH2SO4 ����������Ϊ70%������ ����������Ϊ25%��H2SO4 ���¿����Na2SO3��ĩ ��������(��״���壬������ˮ) ��NaOH��Һ ����ˮ ��Na2CO3��Һ
��ʵ�鿪ʼ�ü���Bƿ�г��ֹ����״���ʡ�������������⣺
(1)A�з�Һ©��ʢ�ŵ��Լ���__________����ƿ��ʢ�ŵ��Լ���__________����ʵ�鿪ʼʱ��Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
(2)B�з�Ӧ�Ļ�ѧ����ʽ��____________________����Ӧ�����������뻹ԭ��������ʵ���֮����__________��
(3)E(���շ�����)��ʢ�ŵ������Լ�������Ӧ�Ļ�ѧ����ʽ��____________________��
(4)���A��E��װ���������ɵ��ٶ���ͬ������Ҳ��ͬ����D�з�����Ӧ�Ļ�ѧ����ʽ��______________________________��
(5)D��������______________________��C��������______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
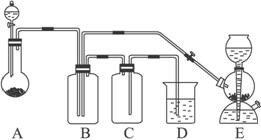
ʵ�����õ����Լ����������Լ���ѡ��
����������Ϊ98%��ŨH2SO4 ����������Ϊ70%������ ����������Ϊ25%��H2SO4 ���¿����Na2SO3��ĩ ������������״���壬������ˮ����NaOH��Һ ����ˮ ��Na2CO3��Һ
��ʵ�鿪ʼ�ü���Bƿ�г��ֹ����״���ʡ�������������⣺
��1��A�з�Һ©��ʢ�ŵ��Լ���__________����ƿ��ʢ�ŵ��Լ���__________����ʵ�鿪ʼʱ��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��2��B�з�Ӧ�Ļ�ѧ����ʽ��______________________����Ӧ�����������뻹ԭ��������ʵ���֮����______________��
��3��E�����շ���������ʢ�ŵ������Լ�������Ӧ�Ļ�ѧ����ʽ��____________________��
��4�����A��E��װ���������ɵ��ٶ���ͬ������Ҳ��ͬ����D�з�����Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��5��D��������________________________��C��������________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ�ϳ���и�����ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ�ʵ����
��14�֣���ͼ���й����仯�����ʵ��װ�ã�
ʵ�����õ���ʵ���Լ���������ѡ��Ũ�����70%�����25%������¿�����������Ʒ�ĩ������������������������Һ������ˮ����̼������Һ��
��ʵ�鿪ʼ���ü���Bƿ�����������ĩ״���ʡ��Իش𣺣��б�ŵı������ţ�
��1��A�з�Һ©��ʢ�ŵ��Լ��� ��
��2��B�з�Ӧ����������ͻ�ԭ��������ʵ������� ��
��3��E�����շ���������ʢ�е������Լ��� ��������Ӧ�����ӷ���ʽ�� ��
��4�����A��E��װ�����巢���ٶ���ͬ������Ҳ��ͬʱ����D�з�Ӧ�����ӷ���ʽ�� ��
��5��D�������� ��C�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ������ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ�ʵ����
��14�֣���ͼ���й����仯�����ʵ��װ�ã�

ʵ�����õ���ʵ���Լ���������ѡ��Ũ�����70%�����25%������¿�����������Ʒ�ĩ������������������������Һ������ˮ����̼������Һ��
��ʵ�鿪ʼ���ü���Bƿ�����������ĩ״���ʡ��Իش𣺣��б�ŵı������ţ�
��1��A�з�Һ©��ʢ�ŵ��Լ��� ��
��2��B�з�Ӧ����������ͻ�ԭ��������ʵ������� ��
��3��E�����շ���������ʢ�е������Լ��� ��������Ӧ�����ӷ���ʽ�� ��
��4�����A��E��װ�����巢���ٶ���ͬ������Ҳ��ͬʱ����D�з�Ӧ�����ӷ���ʽ�� ��
��5��D�������� ��C�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com