
1.52 gͭþ�Ͻ���ȫ�ܽ���50 mL�ܶ�Ϊ1.40 g·mL��1����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������ 1 120 mL(��״��)����Ӧ�����Һ�м���1.0 mol·L��1NaOH��Һ������������ȫ������ʱ���õ�2.54 g����������˵������ȷ����(����)
A���úϽ���ͭ��þ�����ʵ���֮����2��1
B����Ũ������HNO3�����ʵ���Ũ����14.0 mol·L��1
C��NO2��N2O4�Ļ�������У�NO2�����������80%
D���õ�2.54 g����ʱ������NaOH��Һ�������600 mL
�𰸡�D
���������������Ӧ���̣���������غ�˼����������⡣A����Ӧ����Һ�м���NaOH������Mg(OH)2��Cu(OH)2�����������������ӵ���OH��������������n(OH��)��n(e��)����Ͻ���þ��ͭ�����ʵ����ֱ�Ϊx��y������
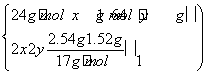
��֮��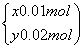 ����úϽ���ͭ��þ�����ʵ���֮��Ϊ2��1��B��������Ũ��c��
����úϽ���ͭ��þ�����ʵ���֮��Ϊ2��1��B��������Ũ��c��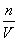 ��
��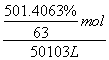 ��14.0 mol·L��1��C��NO2��N2O4���������ᣬ����������NO2�����ʵ���Ϊx�����ݵ����غ��x��(0.05 mol��x)��2��0.06 mol��x��0.04 mol��NO2���������Ϊ80%��D��õ�2.54 g��������Һ�е�����ֻ��NaNO3����n(NaOH)��0.7 mol��0.04 mol��0.02 mol��0.64 mol����NaOH��Һ�������640 mL��
��14.0 mol·L��1��C��NO2��N2O4���������ᣬ����������NO2�����ʵ���Ϊx�����ݵ����غ��x��(0.05 mol��x)��2��0.06 mol��x��0.04 mol��NO2���������Ϊ80%��D��õ�2.54 g��������Һ�е�����ֻ��NaNO3����n(NaOH)��0.7 mol��0.04 mol��0.02 mol��0.64 mol����NaOH��Һ�������640 mL��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں����������������Һ�У�����Fe(NO3)2�������Ȼ�ܹ������������������(����)
A��K����SO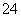 ��NH
��NH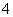 ��CO
��CO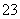 B��K����Ba2����OH����Cl��
B��K����Ba2����OH����Cl��
C��Na����H����Cl����SO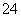 D��Na����Mg2����Cl����NO
D��Na����Mg2����Cl����NO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж��л���ṹ�����ʵ������д������(����)
A������ˮ���뱽�У���ˮ����ɫ��dz���������ڷ����˼ӳɷ�Ӧ
B���������е�6��̼ԭ��֮��ļ���ȫ��ͬ����һ�ֽ���̼̼������̼̼˫��֮��Ķ��صļ�
C������ͱ�ϩ�����ʵ�����1 mol����ȫȼ������3 mol H2O
D��һ�������£�Cl2���ڼױ��ı���������Ϸ���ȡ����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����24 mLŨ��Ϊ0.05 mol·L��1��Na2SO3��Һǡ����20 mLŨ��Ϊ0.02 mol·L��1��K2Cr2O7��Һ��ȫ��Ӧ����֪Na2SO3�ɱ�K2Cr2O7����ΪNa2SO4����Ԫ��Cr�ڻ�ԭ�����еĻ��ϼ�Ϊ(����)
A����2 B����3 C����4 D����5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CuSO4��Һ��K2C2O4��Һ�������һ����ɫ����ˮ����KxCuy(C2O4)z·nH2O��ͨ������ʵ��ȷ���þ������ɡ�(��֪��MnO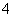 �����������£������ֽܷ�ΪO2��ͬʱ����Mn2����)
�����������£������ֽܷ�ΪO2��ͬʱ����Mn2����)
����a����ȡ0.672 0 g��Ʒ��������ƿ����������2 mol·L��1ϡ���ᣬ��ʹ��Ʒ�ܽ⡣�ټ���30 mLˮ���ȣ���0.200 0 mol·L��1 KMnO4��Һ�ζ����յ㣬����8.00 mL KMnO4��Һ���йط�Ӧ��2MnO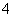 ��5C2O
��5C2O ��16H��===2Mn2����8H2O��10CO2����
��16H��===2Mn2����8H2O��10CO2����
����b�����Ž���Һ��ּ��ȡ���ȴ����pH�����������KI���壬��Һ��Ϊ��ɫ��������ɫ����CuI����0.250 0 mol·L��1 Na2S2O3����Һ�ζ����յ㣬����8.00 mL Na2S2O3��Һ���ζ�ʱ��ӦΪI2��2S2O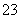 ===2I����S4O
===2I����S4O ��
��
(1)����b�����ɰ�ɫ���������ӷ���ʽ��________________________________________________________________________��
(2)����b�С�����Һ��ּ��ȡ���Ŀ����________________________________________________________________________��
(3)���������ȷ����Ʒ��ɵļ�����̡�
�ټ�����Ʒ��n(C2O )(д���������)
)(��������)
________________________________________________________________________��
�ڼ�����Ʒ��n(Cu2��)(д���������)
________________________________________________________________________��
�۸���________ԭ�������n(K��)��������________ԭ�����n(H2O)��
�ܸ���Ʒ����Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϣ�ͨ������ת�����Ƶ�KClO3���壺
NaCl��Һ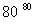 NaClO3��Һ
NaClO3��Һ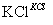 KClO3����
KClO3����
����ɢ��з�Ӧ���ܻ�ѧ����ʽ��NaCl��H2O===NaClO3��__________��
�ڢ���ת���Ļ�����Ӧ������________���÷�Ӧ����������KClO3���������������������ԭ����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�⻯��ͭ(CuH)��һ�ֲ��ȶ����ʣ�����������ȼ�գ�Ҳ�����ᷴӦ����CuSO4��Һ�͡�ij���ʡ���40��50 ��ʱ��Ӧ�ɲ������������й������д������(����)
A����ij���ʡ����л�ԭ��
B��CuH�����ᷴӦ�����ܲ���H2
C��CuH��������ϡ���ᷴӦ��CuH��3H����NO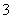 ===Cu2����NO����2H2O
===Cu2����NO����2H2O
D��CuH��������ȼ�գ�CuH��Cl2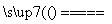 CuCl��HCl
CuCl��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧʵ���Ҳ����ķ�Һ�к���Fe3����Cu2����Ba2����Cl���������ӣ���������з����Է�Һ���д������Ի��ս������Ʊ��Ȼ������Ȼ������塣

(1)����1�к��еĽ���������__________��
(2)����ʱ����H2O2��Һ������Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
(3)���������У�������Ϊ�Լ�X����__________(����ĸ)��
A��BaCl2 B��BaCO3
C��NaOH D��Ba(OH)2
(4)�������2ϴ���Ƿ���ȫ�ķ�����__________��
(5)�Ʊ��Ȼ�������������豣�������������Ŀ����__________��
(6)�ɹ���2�õ�����Һ�Ʊ�BaCl2��ʵ���������Ϊ__________����ȴ�ᾧ��__________��ϴ�ӡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ö��Ե缫���һ��Ũ�ȵ�CuSO4��Һ��ͨ��һ��ʱ��������õ���Һ�м���
0��1 mol Cu(OH)2��ǡ�ûָ������ǰ��Ũ�Ⱥ�pH��������˵����ȷ����(����)
A��������������û����������
B����������ת�Ƶĵ��ӵ����ʵ���Ϊ0.4 mol
C��ԭCuSO4��Һ��Ũ��Ϊ0.1 mol·L��1
D���������������ռ������������Ϊ2.24 L(�����)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com