



| 1.6”Įc(V2-V1 )g |
| 20g |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijÉÕ¼īѳʷŗ¬ÓŠÉŁĮæ²»ÓėĖį×÷ÓƵÄŌÓÖŹ£¬ĪŖĮĖ²ā¶ØĘä“æ¶Č£¬½ųŠŠŅŌĻĀĮ½²½²Ł×÷£ŗ
µŚŅ»²½£ŗÅäÖĘ500 ml ÉÕ¼īѳʷČÜŅŗ”£
£Ø1£©¼ģ²éČŻĮæĘæŹĒ·ńĀ©ŅŗµÄ·½·ØŹĒ£ŗĶłĘæÄŚ¼ÓČėŅ»¶ØĮæĖ®£¬ČūŗĆĘæČū”£
£Ø2£©ÓĆÖŹĮæĪŖ13£®1 gµÄæÕÉÕ±£¬ŠčŅŖ³ĘČ”ÉÕ¼īѳʷ20 g ”£Ōņ·ÅŌŚĶŠÅĢĢģĘ½ÉĻ³ĘČ”Ź±£¬×īÖÕєȔĖłŠčµÄķĄĀėĪŖ””””””””£ØĢīø½±ķÖŠµÄ×ÖÄø£©£¬²¢ŌŚĻĀĶ¼ÖŠŃ”³öÄÜÕżČ·±ķŹ¾ÓĪĀėĪ»ÖƵÄŃ”Ļī””””””””£ØĢī×ÖÄø£©”£
 |
 ø½±ķ£ŗķĄĀė¹ęøń ø½£ŗÓĪĀėĪ»ÖĆ
ø½±ķ£ŗķĄĀė¹ęøń ø½£ŗÓĪĀėĪ»ÖĆ £Ø3£© ÅäÖĘČÜŅŗµÄ²Ł×÷²½ÖčČēĻĀĶ¼µÄŅŅĶ¼ĖłŹ¾£¬Ōņ¼×Ķ¼²Ł×÷Ó¦ŌŚŅŅĶ¼ÖŠµÄ £ØĢīŃ”Ļī×ÖÄø£©Ö®¼ä”£
A£®¢ŁÓė¢Ś”” B£®””¢ŚÓė¢Ū C£®¢ŪÓė¢Ü D£®¢ÜÓė¢Ż

 |
µŚ¶ž²½£ŗÖŠŗĶµĪ¶Ø£¬ŅŌČ·¶ØÉÕ¼īѳʷµÄ“æ¶Č”£
A£®ÓĆ¼īŹ½µĪ¶Ø¹ÜĮæČ”25£®00 mLÉÕ¼īČÜŅŗӌ׶ŠĪĘæÖŠ£¬²¢µĪČė¼øµĪ·ÓĢŖ×÷ÖøŹ¾¼Į
B£®½«ÅضČĪŖc mol / LµÄĮņĖį±ź×¼ČÜŅŗ×°ČėČóĻ“ŗƵÄĖįŹ½µĪ¶Ø¹ÜÖŠ£¬µ÷½ŚŅŗĆęŹ¹æŖŹ¼¶ĮŹżĪŖV1 mL
C£®ŌŚ×¶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½£¬µĪ¶ØÖĮÖÕµćŹ±£¬¼ĒĻĀ¶ĮŹżĪŖV2 mL
ŹŌĢīæÕ£ŗ
£Ø1£©µĪ¶ØÖĮÖÕµćµÄÅŠ¶Ø±ź×¼ŹĒ£ŗµ±¼ÓČė×īŗóŅ»µĪĮņĖįČÜŅŗŹ±£¬ČÜŅŗµÄŃÕÉ«ÓÉ É«±äĪŖ É«”£
£Ø2£©C²½ÖčµÄ²Ł×÷ÖŠ£¬×¶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½µÄ×÷ÓĆŹĒ ”£
£Ø3£©øĆÉÕ¼īѳʷ“æ¶ČĪŖ_________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
¢ń£®ŅŃÖŖ²ā¶ØÖŠŗĶČȵďµŃé²½ÖčČēĻĀ£ŗ¢ŁĮæČ”50mL 0.25mol/LĮņĖįµ¹ČėŠ”ÉÕ±ÖŠ£¬²āĮæĪĀ¶Č ¢ŚĮæČ”50mL 0.55mol/L NaOHČÜŅŗ£¬²āĮæĪĀ¶Č£» ¢Ū½«NaOHČÜŅŗµ¹ČėŠ”ÉÕ±ÖŠ£¬»ģŗĻ¾łŌČŗó²āĮæ»ģŗĻŅŗĪĀ¶Č”£Ēė»Ų“š£ŗ
£Ø1£©NaOHČÜŅŗÉŌ¹żĮæµÄŌŅņ ”£
£Ø2£©¼ÓČėNaOHČÜŅŗµÄÕżČ·²Ł×÷ŹĒ (Ģī×ÖÄø£©”£
A£®ŃŲ²£Į§°ō»ŗĀż¼ÓČė B£®Ņ»“ĪŃøĖŁ¼ÓČė C£®·ÖČż“Ī¼ÓČė
£Ø3£©Ź¹ĮņĖįÓėNaOHČÜŅŗ»ģŗĻ¾łŌȵÄÕżČ·²Ł×÷ŹĒ ”£
£Ø4£©ÉčČÜŅŗµÄĆÜ¶Č¾łĪŖ1g”¤cm-3,ÖŠŗĶŗóČÜŅŗµÄ±ČČČČŻc=4.18J”¤(g”¤”ę)-1£¬Ēėøł¾ŻŹµŃ鏿¾ŻĒó³öÖŠŗĶČČĪŖ”””””””” ”””” Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½_________
£Ø5£©Čō½«ŗ¬0.5 mol H2SO4µÄÅØĮņĖįÓėŗ¬1 mol NaOHµÄČÜŅŗ»ģŗĻ£¬·Å³öµÄČČĮæ £ØĢī”°Š”ÓŚ”±”¢”°µČÓŚ”±»ņ”°“óÓŚ”±£©57.3 kJ£¬ŌŅņŹĒ”””””””””” ””””
¢ņ£®Ä³ÉÕ¼īѳʷŗ¬ÓŠÉŁĮæ²»ÓėĖį×÷ÓƵÄŌÓÖŹ£¬ĪŖĮĖ²ā¶ØĘä“æ¶Č£¬½ųŠŠŅŌĻĀ²Ł×÷£ŗ
A£®ŌŚ250 mLµÄČŻĮæĘæÖŠ¶ØČŻÅä³É250 mLÉÕ¼īČÜŅŗ
B£®ÓĆ¼īŹ½µĪ¶Ø¹ÜĮæČ”25.00 mLÉÕ¼īČÜŅŗӌ׶ŠĪĘæÖŠ£¬²¢µĪČė¼øµĪ·ÓĢŖ×÷ÖøŹ¾¼Į
C£®ŌŚĢģĘ½ÉĻ×¼Č·³ĘČ”ÉÕ¼īѳʷW g£¬ŌŚÉÕ±ÖŠÓĆÕōĮóĖ®Čܽā
D£®½«ĪļÖŹµÄĮæÅضČĪŖMµÄ±ź×¼ĮņĖįČÜŅŗ×°ČėČóĻ“ŗƵÄĖįŹ½µĪ¶Ø¹ÜÖŠ£¬µ÷½ŚŅŗĆęŹ¹æŖŹ¼¶ĮŹżĪŖV1 mL
E.ŌŚ×¶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½£¬µĪ¶ØÖĮČÜŅŗĒ”ŗĆÓÉŗģÉ«±äĪŖĪŽÉ«Ź±£¬¼ĒĻĀ¶ĮŹżĪŖV2 mL
ŹŌĢīæÕ£ŗ
£Ø1£©ÕżČ·²Ł×÷²½ÖčµÄĖ³ŠņŹĒ (ÓĆ×ÖÄø±ķŹ¾£©”£
£Ø2£©¹Ū²ģµĪ¶Ø¹ÜŅŗĆęµÄø߶ȏ±Ó¦×¢Ņā
£Ø3£©E²½ÖčµÄ²Ł×÷֊׶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½µÄ×÷ÓĆŹĒ ”£
£Ø4£©Ä³Ń§ÉśŹµŃ鏱°Ń׶ŠĪĘæÓĆÉÕ¼īѳʷČÜŅŗĻ“µÓ£¬Ź¹²ā¶ØµÄÅضČ_________£ØĢī”°Ę«øß”±”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£©£¬ŌŅņŹĒ
£Ø5£©øĆÉÕ¼īѳʷ“æ¶ČµÄ¼ĘĖćŹ½ĪŖ_________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø16·Ö£©Ä³ÉÕ¼īѳʷŗ¬ÓŠÉŁĮæ²»ÓėĖį×÷ÓƵÄŌÓÖŹ£¬ĪŖĮĖ²ā¶ØĘä“æ¶Č£¬½ųŠŠŅŌĻĀĮ½²½²Ł×÷£ŗ
µŚŅ»²½£ŗÅäÖĘ500 ml ÉÕ¼īѳʷČÜŅŗ”£
£Ø1£©¼ģ²éČŻĮæĘæŹĒ·ńĀ©ŅŗµÄ·½·ØŹĒ£ŗĶłĘæÄŚ¼ÓČėŅ»¶ØĮæĖ®£¬ČūŗĆĘæČū”£
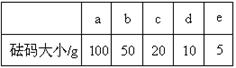
£Ø2£©ÓĆÖŹĮæĪŖ13£®1 gµÄæÕÉÕ±£¬ŠčŅŖ³ĘČ”ÉÕ¼īѳʷ20 g ”£Ōņ·ÅŌŚĶŠÅĢĢģĘ½ÉĻ³ĘČ”Ź±£¬×īÖÕєȔĖłŠčµÄķĄĀėĪŖ””””””””£ØĢīø½±ķÖŠµÄ×ÖÄø£©£¬²¢ŌŚĻĀĶ¼ÖŠŃ”³öÄÜÕżČ·±ķŹ¾ÓĪĀėĪ»ÖƵÄŃ”Ļī””””””””£ØĢī×ÖÄø£©”£
 |
 ø½±ķ£ŗķĄĀė¹ęøń ø½£ŗÓĪĀėĪ»ÖĆ
ø½±ķ£ŗķĄĀė¹ęøń ø½£ŗÓĪĀėĪ»ÖĆ£Ø3£© ÅäÖĘČÜŅŗµÄ²Ł×÷²½ÖčČēĻĀĶ¼µÄŅŅĶ¼ĖłŹ¾£¬Ōņ¼×Ķ¼²Ł×÷Ó¦ŌŚŅŅĶ¼ÖŠµÄ £ØĢīŃ”Ļī×ÖÄø£©Ö®¼ä”£
A£®¢ŁÓė¢Ś”” B£®””¢ŚÓė¢Ū C£®¢ŪÓė¢Ü D£®¢ÜÓė¢Ż

 |
µŚ¶ž²½£ŗÖŠŗĶµĪ¶Ø£¬ŅŌČ·¶ØÉÕ¼īѳʷµÄ“æ¶Č”£
A£®ÓĆ¼īŹ½µĪ¶Ø¹ÜĮæČ”25£®00 mLÉÕ¼īČÜŅŗӌ׶ŠĪĘæÖŠ£¬²¢µĪČė¼øµĪ·ÓĢŖ×÷ÖøŹ¾¼Į
B£®½«ÅضČĪŖc mol / LµÄĮņĖį±ź×¼ČÜŅŗ×°ČėČóĻ“ŗƵÄĖįŹ½µĪ¶Ø¹ÜÖŠ£¬µ÷½ŚŅŗĆęŹ¹æŖŹ¼¶ĮŹżĪŖV1 mL
C£®ŌŚ×¶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½£¬µĪ¶ØÖĮÖÕµćŹ±£¬¼ĒĻĀ¶ĮŹżĪŖV2 mL
ŹŌĢīæÕ£ŗ
£Ø1£©µĪ¶ØÖĮÖÕµćµÄÅŠ¶Ø±ź×¼ŹĒ£ŗµ±¼ÓČė×īŗóŅ»µĪĮņĖįČÜŅŗŹ±£¬ČÜŅŗµÄŃÕÉ«ÓÉ É«±äĪŖ É«”£
£Ø2£©C²½ÖčµÄ²Ł×÷ÖŠ£¬×¶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½µÄ×÷ÓĆŹĒ ”£
£Ø3£©øĆÉÕ¼īѳʷ“æ¶ČĪŖ_________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźøŹĖąŹ”ĢģĖ®Ņ»ÖŠø߶žÉĻѧʌµŚŅ»½×¶Īæ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéĢā£ŗ£Ø±¾Ģā¹²9·Ö£©Ä³ÉÕ¼īѳʷŗ¬ÓŠÉŁĮæ²»ÓėĖį×÷ÓƵÄŌÓÖŹ£¬ĪŖĮĖµĪ¶ØĘä“æ¶Č£¬½ųŠŠŅŌĻĀµĪ¶Ø²Ł×÷£ŗ?
| A£®ŌŚ250 mLµÄČŻĮæĘæÖŠÅäÖĘ250 mLÉÕ¼īČÜŅŗ£» | B£®ÓĆ¼īŹ½µĪ¶Ø¹ÜĮæČ”25.00 mLÉÕ¼īČÜŅŗӌ׶ŠĪĘæÖŠ²¢µĪ¼Ó¼øµĪ·ÓĢŖ×öÖøŹ¾¼Į£»? | C£®ŌŚĢģĘ½ÉĻ×¼Č·³ĘČ”ÉÕ¼īѳʷW””g£¬ŌŚÉÕ±ÖŠÓĆÕōĮóĖ®Čܽā£» | D£®½«ĪļÖŹµÄĮæÅضČĪŖcmol/LµÄ±ź×¼ŃĪĖįČÜŅŗ×°ČėĖįŹ½µĪ¶Ø¹Ü”£µ÷ÕūŅŗĆę¼ĒĻĀæŖŹ¼¶ĮŹżĪŖV1 mL£»E.ŌŚ×¶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½£¬µĪ¶Ø×īŗóŅ»µĪÖĮŗģÉ«øÕŗĆĻūŹ§ ĪŖÖ¹£¬°ė·ÖÖÓŗó²»»Öø“ŗģÉ«£¬¼ĒĻĀ¶ĮŹżV2 mL”£? ĪŖÖ¹£¬°ė·ÖÖÓŗó²»»Öø“ŗģÉ«£¬¼ĒĻĀ¶ĮŹżV2 mL”£? |
 2)¹Ū²ģµĪ¶Ø¹ÜĄļŅŗĆęµÄø߶ȏ±Ó¦×¢Ņā£ŗ ”£?
2)¹Ū²ģµĪ¶Ø¹ÜĄļŅŗĆęµÄø߶ȏ±Ó¦×¢Ņā£ŗ ”£?²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com