
ΓΨΧβΡΩΓΩ‘Ύ2 LΒΡΟή±’»ίΤς÷–”–3÷÷Έο÷ Ϋχ––Ζ¥”ΠΘ§XΓΔYΓΔZΒΡΈο÷ ΒΡΝΩΥφ ±Φδ±δΜ·ΒΡ«ζœΏ»γΆΦΥυ ΨΘ§Ζ¥”Π‘Ύt1min ±¥οΒΫΜ·―ßΤΫΚβΉ¥Χ§ΓΘ

(1) 0ΓΪt1minΡΎΘ§XΒΡ≈®Ε»±δΜ·ΝΩΈΣ___________YΒΡ≈®Ε»±δΜ·ΝΩ______________
(2) 0ΓΪt1minΡΎΘ§YΒΡΤΫΨυΖ¥”ΠΥΌ¬ ΈΣ_________________________________ΓΘ
(3) XΓΔYΓΔZ»ΐ’ΏΒΡΥΌ¬ ÷°±»ΈΣ_____________________ΓΘ
(4) ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_______________________________________ΓΘ
(5) œ¬Ν–ΙΊ”ΎΗΟΖ¥”ΠΒΡΥΒΖ®’ΐ»ΖΒΡ «__________________________________ΓΘ
AΘ°t1min ±Θ§ΗΟΖ¥”Π“―ΆΘ÷Ι
BΘ°t1min÷°«ΑΘ§XΒΡœϊΚΡΥΌ¬ ¥σ”ΎΥϋΒΡ…ζ≥…ΥΌ¬
CΘ°t1min ±Θ§’ΐΖ¥”ΠΥΌ¬ Β»”ΎΡφΖ¥”ΠΥΌ¬
ΓΨ¥πΑΗΓΩ 0.4mol/L 0.6mol/L 0.6/t1molΓΛLΘ≠1ΓΛminΘ≠1 V(X):V(Y):V(Z)=2:3:1 2X![]() 3YΘΪZ BC
3YΘΪZ BC
ΓΨΫβΈωΓΩ(1) ΗυΨίΆΦœώΘ§0ΓΪt1minΡΎΘ§XΒΡ≈®Ε»±δΜ·ΝΩΈΣ![]() =
=![]() =0.4 mol/LΘ§YΒΡ≈®Ε»±δΜ·ΝΩ
=0.4 mol/LΘ§YΒΡ≈®Ε»±δΜ·ΝΩ![]() =
=![]() =0.6 mol/LΘ§Ι ¥πΑΗΈΣΘΚ0.4mol/LΘΜ0.6mol/LΘΜ
=0.6 mol/LΘ§Ι ¥πΑΗΈΣΘΚ0.4mol/LΘΜ0.6mol/LΘΜ
(2) 0ΓΪt1minΡΎΘ§YΒΡΤΫΨυΖ¥”ΠΥΌ¬ ΈΣ![]() =
=![]() =
=![]() mol/(Lmin)Θ§Ι ¥πΑΗΈΣΘΚ
mol/(Lmin)Θ§Ι ¥πΑΗΈΣΘΚ ![]() mol/(Lmin)ΘΜ
mol/(Lmin)ΘΜ
(3) XΓΔYΓΔZ»ΐ’ΏΒΡΥΌ¬ ÷°±»Β»”ΎΈο÷ ΒΡΝΩΒΡ±δΜ·ΝΩ÷°±»=0.8molΘΚ1.2molΘΚ0.4mol=2:3:1Θ§Ι ¥πΑΗΈΣΘΚ2:3:1ΘΜ
(4)ΆΦœσΩ…÷Σ¥”0ΓΪt ±ΩΧΘ§n(X)Φθ–ΓΘ§n(Y)‘ω¥σΘ§n(Z)‘ω¥σΘ§Ω…“‘»ΖΕ®XΈΣΖ¥”ΠΈοΘ§YZΈΣ…ζ≥…ΈοΘ§Γςn(X)ΘΚΓςn(Y)ΘΚΓςn(Z)=0.8ΘΚ1.2ΘΚ0.4=2ΘΚ3ΘΚ1Θ§Υυ“‘Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ2X3Y+ZΘΜΙ ¥πΑΗΈΣΘΚ2X3Y+ZΘΜ
(5)AΘ°Μ·―ßΤΫΚβ «“Μ÷÷Ε·Χ§ΤΫΚβΘ§¥οΒΫΤΫΚβΉ¥Χ§ ±Θ§’ΐΡφΖ¥”ΠΥΌ¬ œύΒ»Θ§ΒΪ≤ΜΈΣ0Θ§Ι A¥μΈσΘΜBΘ°‘Ύt1min÷°«ΑΘ§Ω…ΡφΖ¥”Π…–Έ¥¥οΒΫΜ·―ßΤΫΚβΘ§ΤΫΚβ’ΐœρ“ΤΕ·Θ§‘ρ¥Υ ±v’ΐ(X)ΘΨvΡφ(X)Θ§Ι B’ΐ»ΖΘΜCΘ°‘Ύt1minΘ§Ω…ΡφΖ¥”Π“―¥οΒΫΜ·―ßΤΫΚβΘ§¥Υ ±’ΐΓΔΡφΖ¥”ΠΥΌ¬ œύΒ»Θ§Ι C’ΐ»ΖΘΜΙ ¥πΑΗΈΣΘΚBCΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ”ΟΧζΤ§”κœΓΝρΥαΖ¥”Π÷Τ±Η«βΤχ ±Θ§œ¬Ν–¥κ ©≤ΜΡή Ι«βΤχ…ζ≥…ΥΌ¬ Φ”ΩλΒΡ «Θ® Θ©
AΘ°ΧζΤ§ΗΡΈΣΧζΖέ BΘ°œΓΝρΥαΗΡΈΣ98% ≈®ΝρΥα
CΘ°Ε‘ΗΟΖ¥”ΠΧεœΒΦ”»» DΘ°ΧζΤ§ΗΡΈΣ–ΩΤ§
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΧΦΦΑΤδΜ·ΚœΈο‘ΎΜ·ΙΛ…ζ≤ζ÷–”–Ή≈ΙψΖΚΒΡ”Π”ΟΓΘ
I.ΈΣΫβΨω¥σΤχ÷–CO2ΒΡΚ§ΝΩ‘ω¥σΒΡΈ ΧβΘ§Ρ≥ΩΤ―ßΦ“Χα≥ω»γœ¬ΙΙœκΘΚ
Α―ΙΛ≥ß≈≈≥ωΒΡΗΜΚ§CO2ΒΡΖœΤχΨ≠ΨΜΜ·¥Β»κΧΦΥαΦΊ»ή“ΚΈϋ ’Θ§»ΜΚσ‘ΌΑ―CO2¥”»ή“Κ÷–Χα»Γ≥ωά¥Θ§‘ΎΚœ≥…Υΰ÷–Ψ≠Μ·―ßΖ¥”Π ΙΖœΤχ÷–ΒΡCO2ΉΣ±δΈΣ»ΦΝœΦΉ¥ΦΓΘ
≤ΩΖ÷ΦΦ θΝς≥Χ»γœ¬:
Δ≈Κœ≥…Υΰ÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________ΘΜΓςH<0ΓΘΗΟΖ¥”ΠΈΣΩ…ΡφΖ¥”ΠΘ§¥”ΤΫΚβ“ΤΕ·‘≠άμΖ÷ΈωΘ§ΒΆΈ¬”–άϊ”ΎΧαΗΏ‘≠ΝœΤχΒΡΤΫΚβΉΣΜ·¬ ΓΘΕχ ΒΦ …ζ≤ζ÷–≤…”Ο300ΓφΒΡΈ¬Ε»Θ§≥ΐΩΦ¬«Έ¬Ε»Ε‘Ζ¥”ΠΥΌ¬ ΒΡ”ΑœλΆβΘ§ΜΙ÷ς“ΣΩΦ¬«ΝΥ___________________________________________________________________ΓΘ
(2)¥”Κœ≥…ΥΰΖ÷άκ≥ωΦΉ¥ΦΒΡ‘≠άμ”κœ¬Ν–_______≤ΌΉςΒΡ‘≠άμ±»ΫœœύΖϊ(ΧνΉ÷ΡΗΘ©
A.Ιΐ¬Υ B.Ζ÷“Κ C.’τάΓ D.ΫαΨß
(3)»γΫΪCO2”κH2“‘1:4ΒΡΧεΜΐ±»ΜλΚœΘ§‘Ύ Β±ΒΡΧθΦΰœ¬Ω…÷ΤΒΟCH4ΓΘ–¥≥ωCO2(g)”κH2(g)Ζ¥”Π…ζCH4(g)”κ“ΚΧ§Υ°ΒΡ»»Μ·―ßΖΫ≥Χ Ϋ:
“―÷ΣΘΚCH4(g)+2O2(g)=CO2(g)+2H2O(l) ΓςH1=-890.3kJ/mol
2H2(g)+O2(g)=2H2O(l) ΓςH2=-571.6kJ/mol
_______________________________________________________________________ΓΘ
II.ΦΉΆι»Φ…’ΜαΖ≈≥ω¥σΝΩΒΡ»»Θ§Ω…ΉςΈΣΡή‘¥”Π”Ο”Ύ»ΥάύΒΡ…ζ≤ζΚΆ…ζΜνΓΘ
ΦΚ÷ΣΘΚΔΌ2CH4(g)+3O2(g)=2CO(g)+4H2O(l)ΘΜ ΓςH1=-1214.6kJ/mol
ΔΎCO2(g)=CO(g)+1/2O2(g)ΘΜ ΓςH2=+283.0kJ/mol
‘ρ±μ ΨΦΉΆι»Φ…’»»ΒΡ»»Μ·―ßΖΫ≥Χ Ϋ______________________________ΓΘ
III.Ρ≥–Υ»Λ–ΓΉιΡΘΡβΙΛ“ΒΚœ≥…ΦΉ¥ΦΒΡΖ¥”Π:CO(g)+2H2(g)![]() CH3OH(g)Θ§‘Ύ»ίΜΐΙΧΕ®ΈΣ2LΒΡΟή±’»ίΤς÷–≥δ»κ1mol CO 2mol H2Θ§Φ”»κΚœ ΒΡ¥ΏΜ·ΦΝΘ®¥ΏΜ·ΦΝΧεΜΐΚω¬‘≤ΜΦΤΘ©ΚσΩΣ ΦΖ¥”ΠΓΘ≤βΒΟ»ίΤςΡΎΒΡ―Ι«ΩΥφ ±Φδ±δΜ·»γœ¬:
CH3OH(g)Θ§‘Ύ»ίΜΐΙΧΕ®ΈΣ2LΒΡΟή±’»ίΤς÷–≥δ»κ1mol CO 2mol H2Θ§Φ”»κΚœ ΒΡ¥ΏΜ·ΦΝΘ®¥ΏΜ·ΦΝΧεΜΐΚω¬‘≤ΜΦΤΘ©ΚσΩΣ ΦΖ¥”ΠΓΘ≤βΒΟ»ίΤςΡΎΒΡ―Ι«ΩΥφ ±Φδ±δΜ·»γœ¬:
±Φδ/min | 0 | 5 | 10 | 15 | 20 | 25 |
―Ι«Ω/Mpa | 12.6 | 10.8 | 9.5 | 8.7 | 8.4 | 8.4 |
(1)¥”Ζ¥”ΠΩΣ ΦΒΫ20min ±Θ§“‘CO±μ ΨΖ¥”ΠΥΌ¬ ΈΣ_____________________ΓΘ
(2)œ¬Ν–Οη ωΡήΥΒΟςΖ¥”Π¥οΒΫΤΫΚβΒΡ «_______________________
A.ΉΑ÷ΟΡΎΤχΧε―’…Ϊ≤Μ‘ΌΗΡ±δ B.»ίΤςΡΎΤχΧεΒΡΤΫΨυΡΠΕϊ÷ ΝΩ±Θ≥÷≤Μ±δ
C.»ίΤςΡΎΤχΧεΒΡ―Ι«Ω±Θ≥÷≤Μ±δ D.»ίΤςΡΎΤχΧεΟήΕ»±Θ≥÷≤Μ±δ
(3)ΗΟΈ¬Ε»œ¬ΤΫΚβ≥Θ ΐK=____Θ§»τ¥οΒΫΤΫΚβΚσΦ”»κ…ΌΝΩCH3OH(g)Θ§¥Υ ±ΤΫΚβ≥Θ ΐK÷ΒΫΪ____ (ΧνΓΑ‘ω¥σΓ±ΓΔΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)
(4)ΗΟΖ¥”Π¥οΒΫΤΫΚβΚσΘ§‘Όœρ»ίΤς÷–≥δ»κ1mol CO 2mol H2Θ§¥Υ ±COΒΡΉΣΜ·¬ ΫΪ__________(ΧνΓΑ‘ω¥σΓ±ΓΔΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ25 Γφ ±Θ§œρ¥ΩΥ°÷–Φ”»κNaOHΘ§ Ι»ή“ΚΒΡpHΈΣ11Θ§‘ρ”…NaOHΒγάκ≥ωΒΡOHΘ≠άκΉ”≈®Ε»”κΥ°ΒγάκΒΡOHΘ≠άκΉ”≈®Ε»÷°±»ΈΣ
A. 1010ΓΟ1 B. 5ΓΝ109ΓΟ1 C. 108ΓΟ1 D. 1ΓΟ1
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–Έο÷ ÷–Θ§ τ”ΎΧ«άύΒΡ «
A.œΥΈ§ΥΊB.÷≤Έο”ΆC.ΒΑΑΉ÷ D.Ψέ““œ©
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“―÷Σœ¬Ν–»»Μ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ 2H2(g)ΘΪO2(g)===2H2O(l)ΠΛHΘΫΘ≠570.0 kJΓΛmolΘ≠1
ΔΎH2O(g)===H2(g)ΘΪ![]() O2(g)ΠΛHΘΫ +241.8 kJΓΛmolΘ≠1
O2(g)ΠΛHΘΫ +241.8 kJΓΛmolΘ≠1
ΔέC(s)ΘΪ![]() O2(g)===CO(g)ΠΛHΘΫΘ≠110.5 kJΓΛmolΘ≠1
O2(g)===CO(g)ΠΛHΘΫΘ≠110.5 kJΓΛmolΘ≠1
ΔήC(s)ΘΪO2(g)===CO2(g) ΠΛHΘΫΘ≠393.5 kJΓΛmolΘ≠1
ΜΊ¥πœ¬Ν–ΗςΈ ΧβΘΚ
(1)…œ ωΖ¥”Π÷– τ”ΎΖ≈»»Ζ¥”ΠΒΡ «___________________ΓΘ(Χν–ρΚ≈Θ©
(2)H2ΒΡ»Φ…’»»ΠΛHΘΫ___________________ΘΜCΒΡ»Φ…’»»ΠΛHΘΫ___________________ΓΘ
(3)»Φ…’10 g H2…ζ≥…“ΚΧ§Υ°Θ§Ζ≈≥ωΒΡ»»ΝΩΈΣ___________________ΓΘ
(4)»γΙϊ–η“Σ ΆΖ≈787kJΒΡ»»ΝΩΘ§–η“ΣΆξ»Ϊ»Φ…’____________________gΒΡΧΦΓΘ
(5)COΒΡ»Φ…’»»ΠΛHΘΫ____Θ§Τδ»»Μ·―ßΖΫ≥Χ ΫΈΣ________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ‘ΎΟή±’»ίΤς÷–Θ§“ΜΕ®ΧθΦΰœ¬Ϋχ––»γœ¬Ζ¥”ΠΘΚNO (g) +CO(g)![]()
![]() N2(g) +CO2 (g) ΓςH=-373.2 kJ/mol¥οΒΫΤΫΚβΚσΘ§ΈΣΧαΗΏΗΟΖ¥”ΠΒΡΥΌ¬ ΚΆNOΒΡΉΣΜ·¬ Θ§≤…»ΓΒΡ’ΐ»Ζ¥κ © «Θ® Θ©
N2(g) +CO2 (g) ΓςH=-373.2 kJ/mol¥οΒΫΤΫΚβΚσΘ§ΈΣΧαΗΏΗΟΖ¥”ΠΒΡΥΌ¬ ΚΆNOΒΡΉΣΜ·¬ Θ§≤…»ΓΒΡ’ΐ»Ζ¥κ © «Θ® Θ©
A. Φ”¥ΏΜ·ΦΝΆ§ ±…ΐΗΏΈ¬Ε» B. Φ”¥ΏΜ·ΦΝΆ§ ±‘ω¥σ―Ι«Ω
C. …ΐΗΏΈ¬Ε»Ά§ ±≥δ»κN2 D. ΫΒΒΆΈ¬Ε»Ά§ ±‘ω¥σ―Ι«Ω
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΜ·―ß”κ…ζΜνœΔœΔœύΙΊΓΘœ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «
A.”Ά÷§Ω…“‘÷ΤΖ ‘μ
B.ΒμΖέΟΜ”–ΧπΈΕΘ§ΒμΖέ≤Μ τ”ΎΧ«άύ
C.ΝΗ ≥ΡπΨΤΙΐ≥Χ…φΦΑΥ°ΫβΓΔΖ÷ΫβΒ»Ζ¥”Π
D.ΨΏ”–«Ω―θΜ·–‘ΒΡΚ§¬»œϊΕΨ“ΚΩ… Ι–¬ΙΎ≤ΓΕΨ±δ–‘ ßΜν
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩK2Cr2O7»ή“Κ÷–¥φ‘ΎΤΫΚβΘΚCr2O72-(≥»…Ϊ) ![]() 2CrO42-(ΜΤ…Ϊ)+2H+ ΓΘ”ΟK2Cr2O7»ή“ΚΫχ––œ¬Ν– Β―ιΘ§ΫαΚœ Β―ιΘ§œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «Θ® Θ©
2CrO42-(ΜΤ…Ϊ)+2H+ ΓΘ”ΟK2Cr2O7»ή“ΚΫχ––œ¬Ν– Β―ιΘ§ΫαΚœ Β―ιΘ§œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «Θ® Θ©
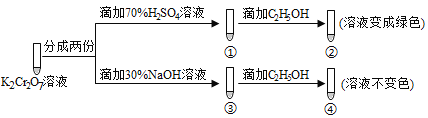
A. ΔΌ÷–»ή“Κ≥»…ΪΦ”…νΘ§Δέ÷–»ή“Κ±δΜΤ B. ΔΎ÷–Cr2O72-±ΜC2H5OHΜΙ‘≠
C. »τœρΔή÷–Φ”»κ70%H2SO4»ή“Κ÷ΝΙΐΝΩΘ§»ή“Κ±δΈΣ≥»…Ϊ D. Ε‘±»ΔΎΚΆΔήΩ…÷ΣK2Cr2O7Υα–‘»ή“Κ―θΜ·–‘«Ω
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com