
þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ�ijѧУ�о���ѧϰС��Ӻ�ˮɹ�κ����±����Ҫ��Na+��Mg2+��Cl����Br���ȣ���ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺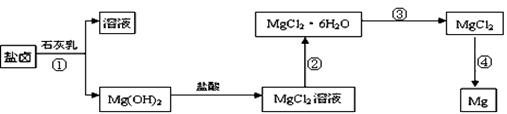
��1���ӹ��̢ٵõ���Mg��OH��2�����л���������Ca��OH��2 ����ȥ����Ca��OH��2�ķ������Ƚ��������뵽ʢ�� ������Һ���ձ��У���ֽ���� �� ��������������ɵô�����Mg��OH��2��д���йط�Ӧ�����ӷ���ʽ ��
��2����ͼ�Ǹ�У�о���ѧϰС����ƽ��й��̢۵�ʵ��װ��ͼ������װ��A�������� ����Ҫ��֤������ˮMgCl2�в���NaCl����IJ��������� ��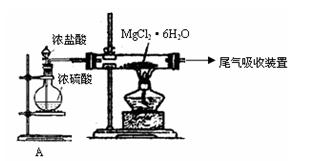
��3��д�����̢��з�����Ӧ�Ļ�ѧ����ʽ ���ù��̵õ���þ������Ҫ���ض���ѭ������ȴ��Ӧѡ�� �����Լ����ƣ�����ȴ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
| FeBr3 |
| FeBr3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
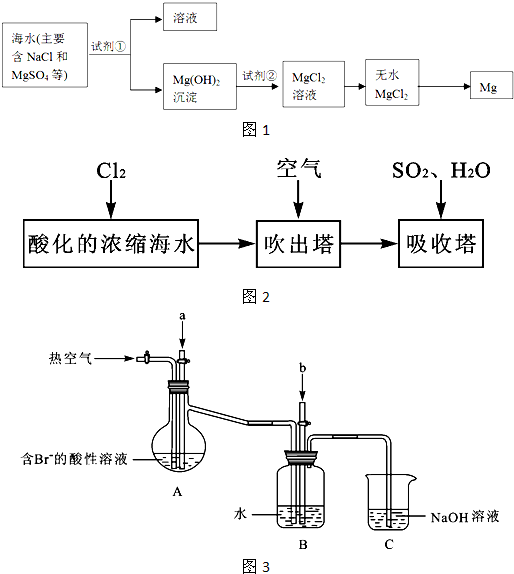
| �� |
| ��� |
| 2800�� |
| �� |
| C |
| ��ԭ |
| HCl |
| ||
| 714�� |
| HCl |
| ���ý��� |
| ��ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
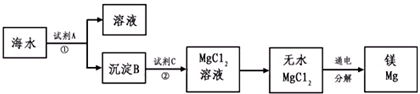
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com