
��ҵ�Ϻϳɰ����ȷ�Ӧ����ʽ���£�N2(g)+3H2(g) 2NH3(g) ��H����92 kJ/mol
2NH3(g) ��H����92 kJ/mol
��1������֪�ƻ�1mol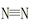 ����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��
����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��
��2���ں��º�ѹ�������£���2mol N2��6molH2ͨ��һ�ݻ��ɱ�������з�Ӧ���ﵽƽ�����������Ϊ��Ӧǰ��75%����ù����ͷŵ�����Ϊ kJ��������ת����Ϊ ��ƽ�����ռ���������������Ϊ ��
��3������1mol N2��1molH2ͨ��������ͬ������ܱ����������У������������¶Ⱥ�������䣬�����������¶Ⱥ�ѹǿ���䣬����һ��ʱ������������ﵽƽ��״̬��
�ٽ���ƽ�������ʱ�䣺�� �ң������,������������
�ڴﵽƽ������������������ �ң������,������������
��1�� 391��2�� 92��50%�� ��33.3% ��3���� �� �� ��
��33.3% ��3���� �� �� ��
���������������1����Ӧ�ȵ��ڷ�Ӧ����ܼ��ܼ�ȥ��������ܼ��ܣ���N��H����Ϊ xkJ/mol����945.6kJ/mol��3��436 kJ/mol��6��x kJ/mol����92.2kJ/mol�����x��391��
��2�� N2(g)+3H2(g) 2NH3(g)
2NH3(g)
��ʼ����mol�� 2 6 0
ת������mol�� x 3x 2x
ƽ������mol�� 2��x 6��3x 2x
����ݴﵽƽ�����������Ϊ��Ӧǰ��75%��֪ ��0.75
��0.75
���x��1
���Ըù����ͷŵ�����Ϊ92kJ
������ת����Ϊ ��100%��50%
��100%��50%
ƽ�����ռ���������������Ϊ =
=
��3���ٸ��ݷ���ʽN2(g)+3H2(g) 2NH3(g)��֪������Ӧ�������С�Ŀ��淴Ӧ��������������ݻ����䣬��ѹǿ���ͣ���˵���ڷ�Ӧ�������������е�ѹǿʼ�մ��ڼ������е�ѹǿ��ѹǿ��Ӧ���ʿ죬����ƽ���ʱ���٣�������ƽ�������ʱ�䣺�ף��ҡ�
2NH3(g)��֪������Ӧ�������С�Ŀ��淴Ӧ��������������ݻ����䣬��ѹǿ���ͣ���˵���ڷ�Ӧ�������������е�ѹǿʼ�մ��ڼ������е�ѹǿ��ѹǿ��Ӧ���ʿ죬����ƽ���ʱ���٣�������ƽ�������ʱ�䣺�ף��ҡ�
������Ӧ�������С�Ŀ��淴Ӧ�����ѹǿ��������ƽ��������Ӧ�����ƶ�����������������������Դﵽƽ�����������������ף��ҡ�
���㣺���鷴Ӧ�ȵļ��㡢���淴Ӧ���йؼ����Լ����������ƽ��״̬�ͷ�Ӧ���ʵ�Ӱ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ҵ�������һ������ʳ������ֲ����ά�ӹ��ɵ�ȼ���Ҵ�����ͨ���Ͱ�һ�����������γɵ����������Դ�������ҹ��Ĺ��ұ����Ҵ���������90%����ͨ������10%���Ҵ����Ͷ��ɡ�
��1������ʳ�����ֲ����ά�ɵõ������ǣ�д���������Ƶ��Ҵ��Ļ�ѧ����ʽ: ��
��2���ڳ��³�ѹ�£�1gC2H5OH��ȫȼ������CO2��Һ̬H2Oʱ�ų�29.71 kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3����ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺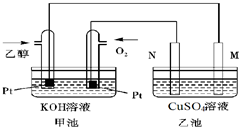
�ټ����Ҵ���Pt�缫�ĵ缫��ӦʽΪ_________________________��
���ڹ��������У��ҳ������缫���ռ�����״����224mL����ʱ���׳����������������������Ϊ mL(��״����)������ʱ�ҳ���Һ���Ϊ200mL�����ҳ�����Һ��pHΪ ��
����Ҫʹ�����ҳص���Һ��ȫ�ָ�����ʼ״̬�������ҳ��м��� (�����)
| A��0.01molCu |
| B��0.01molCuO |
| C��0.01molCu(OH)2 |
| D��0.01molCuCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11�֣��о�ȼ�ϵ�ȼ�պͶ���Ⱦ���������������������ڷ�ֹ������Ⱦ����Ҫ���塣
��1����úת��Ϊ�������ȼ�ϣ�
��֪��H2(g)+1/2O2(g)=H2O(g)  H= ?241��8kJ/mol
H= ?241��8kJ/mol
C(s)+1/2O2(g)=CO(g)  H= ?110��5kJ/mol
H= ?110��5kJ/mol
д����̿��ˮ������Ӧ��H2��CO���Ȼ�ѧ����ʽ ��
��2��һ�������£����ܱ������ڣ�SO2��������SO3���Ȼ�ѧ����ʽΪ��2SO2(g)+O2(g) 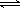 2SO3(g)��
2SO3(g)��
��H=?a kJ/mo1������ͬ������Ҫ��õ�2akJ��������������ʵ����ʵ���������
A��4mo1 SO2��2mol O2���������� B��4mol SO2��2mo1 O2��2mol SO3
C��4mol SO2��4mo1 O2������ D��6mo1 SO2��4mo1 O2
��3������β����NOx��CO�����ɼ�ת����
����֪����������NO�ķ�ӦΪ��N2(g)+O2(g)  2NO(g)
2NO(g)  H��0
H��0
��һ���¶��µĶ����ܱ������У���˵���˷�Ӧ�Ѵ�ƽ�����
A��ѹǿ���� B���������ƽ����Է�����������
C��2v��(N2)��v��(NO) D�� N2������������ٸı�
������ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO(g)=2C(s)+O2(g)  H��0��
H��0��
�����������ܷ�ʵ�ֵ����� ��
��4��ȼ��CO��H2��һ�������¿����ת����CO(g)��H2O(g)  CO2(g)��H2(g)����420��ʱ��ƽ�ⳣ��K=9������Ӧ��ʼʱ��CO��H2O��Ũ�Ⱦ�Ϊ0��1mol/L����CO�ڴ˷�Ӧ�����µ�ת����Ϊ ��
CO2(g)��H2(g)����420��ʱ��ƽ�ⳣ��K=9������Ӧ��ʼʱ��CO��H2O��Ũ�Ⱦ�Ϊ0��1mol/L����CO�ڴ˷�Ӧ�����µ�ת����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g)��H���D24��8kJ?mol-1
��3Fe2O3(s)+CO(g)=2Fe3O4(s)+CO2(g)��H���D47��2kJ?mol-1
��Fe3O4(s)+CO(g)=3FeO(s)+CO2(g)��H��+640��5kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��__________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��������ͨ����������S8(б����)����ʽ���ڣ���������״̬ʱ������S2��S4��S6��S8�ȶ���ͬ�������壬����S4��S6��S8�������ƵĽṹ�ص㣬��ṹ����ͼ��ʾ��
��һ�������£�S8(s)��O2(g)������Ӧ����ת��ΪSO2(g)��SO3(g)����Ӧ���̺�������ϵ������ͼ��ʾ(ͼ�еĦ�H��ʾ����1 mol������������)��
(1)д����ʾS8ȼ���ȵ��Ȼ�ѧ����ʽ_____________________��
(2)д��SO3�ֽ�����SO2��O2���Ȼ�ѧ����ʽ_________________________��
(3)����֪SO2���������ļ���Ϊd kJ��mol-1��O2���������ļ���Ϊe kJ��mol-1����S8������������ļ���Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2011��11��1�գ��ҹ��������Ƶġ���������F��ң�����ػ���������۰˺š��ɴ�����̫��Ԥ�������������롰�칬һ�š�Ŀ�������ʵ�ֳɹ��Խӡ�ƫ�����£�C2H8N2)������������(N2O4)�dz���ϵ�л���ij����ƽ�������ش�����������⣺
��1��ƫ�����£�C2H8N2)������������(N2O4)��Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ��
C2H8N2��2N2O4 3N2��2X��4H2O
3N2��2X��4H2O
��X�Ļ�ѧʽΪ____________��ѡ������ѡ��ı����ĸ����
A��O2 B��CO2 C��H2
��2��1 gҺ̬ƫ��������������Һ̬������������ȫ��Ӧ������̬����ų�Q kJ����������ͬ������0.1 molƫ�����·����÷�Ӧ�ܷų�������Ϊ_____________kJ (ѡ�����б����ĸ)��
A��6Q B��30Q C��60Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)(1)��(N2H4)�Ƿ��亽��ɴ����õĸ���ȼ�ϡ���NH3��NaClO��һ�����ʵ����Ȼ�Ϸ�Ӧ�������¡�NaCl��ˮ���÷�Ӧ�Ļ�ѧ����ʽ��_____________________________��
(2)�ڻ���ƽ�����װ��ǿ��ԭ����(N2H4)��ǿ�������������⣬�����ǻ��ʱ���������������壬���ų������ȡ���֪��H2O(l)H2O(g) ��H=" +44" kJ/mol��12.8 gҺ̬���������������ⷴӦ���ɵ�����ˮ�������ų�256.65 kJ��������
����д��Һ̬����������ⷴӦ����Һ̬ˮ���Ȼ�ѧ����ʽ______________________��
����16 gҺ̬���������������ⷴӦ����Һ̬ˮʱ�ų���������___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪��2NO2(g) N2O4(g)����H��0���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ����ͼ��ʾ��
N2O4(g)����H��0���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ����ͼ��ʾ��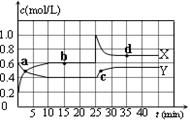
��a��b��c��d�ĸ����У���ʾ��ѧ��Ӧ����ƽ��״̬�ĵ��� ��
��ǰ10 min����N��2��ʾ�Ļ�ѧ��Ӧ����v(N��2)�� mol��L-1��min-1����Ӧ�ڵ�һ��ƽ����ƽ�ⳣ��K(1)�� �����÷�����ʾ������Ӧ�ڵڶ���ƽ����ƽ�ⳣ��K(2)���һ��ƽ����ƽ�ⳣ��K(1)�Ĺ�ϵ��K(2) K(1)�����������������������
��������ͼ�����л���1 mol N2O4ͨ��2L���ܱ������з�Ӧ���������е������仯ʾ��ͼ�����������Ϸֱ�����Ӧ���������Ļ�ѧʽ��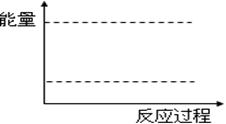
��2����ͼ��a��b��c��d�ֱ��������Ԫ�أ���A�壩��Te���ڣ���Se��������S�����⻯��ķ�Ӧ�ȵ�����ʾ��ͼ���Իش��������⣺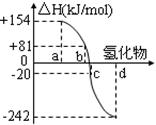
��������ɣ��ǽ���Ԫ���⻯����ȶ������γ��⻯��ķ�Ӧ�ȡ�H�Ĺ�ϵ ��
��д�������ⷢ���ֽⷴӦ���Ȼ�ѧ��Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ú�ˮ��Դ���л��������IJ��ֹ���������ͼ��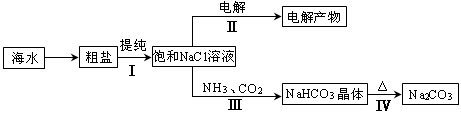
��1������I�У�����ȥ�����к��е�Ca2����Mg2����SO42�������ӣ��轫�����ܽ�������ҩƷ���г��������˵ȡ�����ҩƷ�Ͳ�����˳������� ��
a��Na2CO3��NaOH��BaCl2�����ˡ����� b��NaOH��BaCl2��Na2CO3�����ˡ�����
c��NaOH��Na2CO3��BaCl2�����ˡ����� d��BaCl2��Na2CO3��NaOH�����ˡ�����
��2������II�У���ⱥ��NaCl��Һ�����ӷ���ʽΪ ��ͨ�翪ʼ�������������������� ������������ҺpH�� �����������С�����䡱����
��3������III�У�ͨ����Ӧ�õ�NaHCO3���塣��ͼΪNaCl��NH4Cl��NaHCO3��NH4HCO3���ܽ�����ߣ������ܱ�ʾNaHCO3�ܽ�����ߵ��� ����ѧ��Ӧ����ʽ�� ��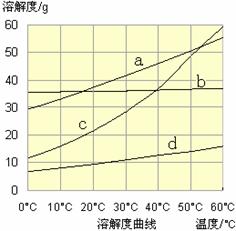
��4������IV�У����ô�������������������ʣ��ᴿ���Ĺ������£���̼������Ʒ������ˮ�ܽ⡢���� ���� �����ˡ�ϴ��2-3�Σ��õ�����Na2CO3?10H2O��Na2CO3?10H2O��ˮ�õ���ˮ̼���ƣ���֪��
Na2CO3��H2O(s)==Na2CO3(s)+H2O(g) ��H1=+58.73kJ��mol��1
Na2CO3��10H2O(s)==Na2CO3��H2O(s)+9H2O(g) ��H2=" +473.63" kJ��mol��1
�Ѹù��̲�������̬ˮ��ȫҺ���ͷŵ�����ȫ����������Na2CO3������ܺģ�������������ʧ����������1molNa2CO3��Ҫ����92.36kJ���ɴ˵ó���H2O(g��==H2O(l) ��H =�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com