
���� �ٴ�����Һ��̼�������ˮ���Լ��ԣ�����������Һ������������ˮ���õ�����Ϊ̼���ƣ�
��A��������Һ�������غ������n��Na��=n��C����
B����Һ�д��������غ㣬c��H+��ˮ=c��OH-��ˮ��
C��̼������Һ��̼�������ˮ��ʼ��ԣ�
D��̼������Һ��̼������ӷֲ�ˮ��ʼ���
��� �⣺�ٴ�����ǿ�������Σ�̼��������ܷ���ˮ��ʹ��Һ�е����������ӵ�Ũ�ȴ��������ӵ�Ũ�ȣ�������Һ�ʼ��ԣ����ӷ���ʽΪ��CO32-+H2O?HCO3-+OH-������������Һ������������ˮ��ˮ��ƽ��������еõ�����Ϊ̼���ƣ�
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��Na2CO3��
��A��������Һ�������غ������n��Na��=n��C����c��Na+��=2c��HCO3-��+2c��CO32-��+2c��H2CO3������A����
B����Һ�д��������غ㣬c��H+��ˮ=c��OH-��ˮ��c��OH-��=c��H+��+c��HCO3-��+2c��H2CO3������B��ȷ��
C��̼������Һ��̼�������ˮ��ʼ��ԣ���Һ������Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��OH-����c��H+������C����
D��̼������Һ��̼������ӷֲ�ˮ��ʼ��ԣ�����Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��OH-����c��HCO3-������D��ȷ��
�ʴ�Ϊ��BD��
���� ���⿼��������ˮ���Ӧ�ã�Ҫע�⣺����ˮ�������ģ����������ӵ��Σ����������γ��⣩��ˮ��Һ���Ե���Ϊ������Ԫ��������ӷֲ�ˮ�⣬�Ե�һ��Ϊ������Һ������Ũ�ȴ�С�ȽϷ����жϣ���Ŀ�Ѷ��еȣ�


 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
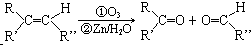

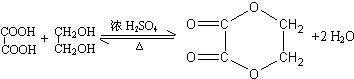 ����������2���Ȼ�������ʹBr2��CCl4��Һ��ɫ��G��ͬ���칹����HOOC-CH=CH-COOH��
����������2���Ȼ�������ʹBr2��CCl4��Һ��ɫ��G��ͬ���칹����HOOC-CH=CH-COOH��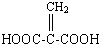 ��д�ṹ��ʽ����
��д�ṹ��ʽ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
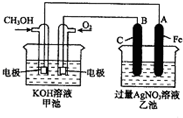 ��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O
��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

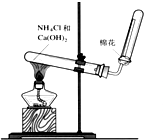
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
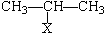 ��
�� CH=CH2�����ϩ�Ĺ�������һ�ָ߷�����ȼ�������еͶ������ȶ��Ժõ��ŵ㣮
CH=CH2�����ϩ�Ĺ�������һ�ָ߷�����ȼ�������еͶ������ȶ��Ժõ��ŵ㣮 ��
��
 CHBrCH3��
CHBrCH3�� CHBrCH3����������������Һ���ȵõ�A���õ�Aʱ��������û�л�ѧ���Ķ��Ѻ����ɣ�
CHBrCH3����������������Һ���ȵõ�A���õ�Aʱ��������û�л�ѧ���Ķ��Ѻ����ɣ�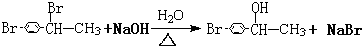 ��
��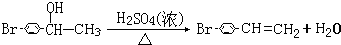 ��
��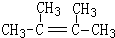 ��1molB��ȫȼ��ʱ����9mol
��1molB��ȫȼ��ʱ����9mol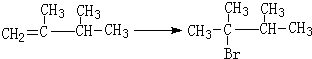
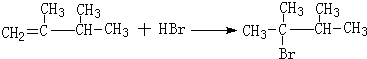
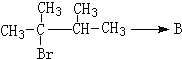
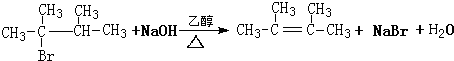 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
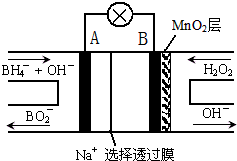 ����NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ��ʾ���õ���ܷ�Ӧ����ʽ��NaBH4+4H2O2�TNaBO2+6H2O���йص�˵������ȷ���ǣ�������
����NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ��ʾ���õ���ܷ�Ӧ����ʽ��NaBH4+4H2O2�TNaBO2+6H2O���йص�˵������ȷ���ǣ�������| A�� | �缫BΪ����������MnO2������������ԭ��صĹ���Ч�� | |
| B�� | �ŵ�����У�Na+������������Ǩ�� | |
| C�� | ��ظ����ĵ缫��ӦΪ��BH4-+8OH--8e-�TBO2-+6H2O | |
| D�� | �ڵ�ط�Ӧ�У�ÿ����1L 6mol/L H2O2��Һ��������������·�еĵ���Ϊ12mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��ˮ��������п������ | B�� | ��ˮ���������Դ�ĸ������� | ||
| C�� | ��ˮ���������Դ���������� | D�� | ���ж���˹����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
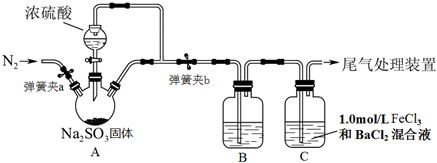
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com