
COCl2�׳ƹ��������ж����塣��һ�������£��ɷ����Ļ�ѧ��ӦΪ��
COCl2(g) CO(g)��Cl2(g)����H<0 �����й�˵����ȷ����(����)
A����һ�������£�ʹ�ô����ܼӿ췴Ӧ���ʲ���߷�Ӧ���ƽ��ת����
B������Ӧ��ƽ��ʱ�����º�ѹ������ͨ��Ar�������COCl2��ת����
C����λʱ��������CO��Cl2�����ʵ���֮��Ϊ1��1ʱ����Ӧ�ﵽƽ��״̬
D��ƽ��ʱ�������������䣬�����¶ȿ�ʹ�÷�Ӧ��ƽ�ⳣ������
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
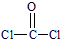
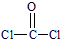
 �ۼ���
�ۼ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��ׯ��ģ��̼�������ȡ���������������������ϢϢ��صĻ�ѧԪ�أ�
��2012?��ׯ��ģ��̼�������ȡ���������������������ϢϢ��صĻ�ѧԪ�أ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
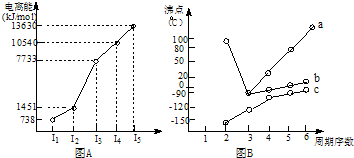
| �� | CH4 | CH3CH3 | CH3��CH2��2CH3 | �������� | 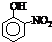 |
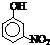 |
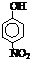 |
| �е�/�� | -164 | -88.6 | -0.5 | �۵�/�� | 45 | 96 | 114 |
| 2 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com