
 ŌŖĖŲÖÜĘŚ±ķµÄÅÅĮŠŗĶ·ÖĒųÓėŌŖĖŲ»łĢ¬Ō×ÓŗĖĶāµē×ÓµÄÅŲ¼Ļ¢Ļ¢Ļą¹Ų£¬Ēė¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ
ŌŖĖŲÖÜĘŚ±ķµÄÅÅĮŠŗĶ·ÖĒųÓėŌŖĖŲ»łĢ¬Ō×ÓŗĖĶāµē×ÓµÄÅŲ¼Ļ¢Ļ¢Ļą¹Ų£¬Ēė¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö £Ø1£©øł¾Żø÷ÖÜĘŚČŻÄÉŌŖĖŲÖÖŹż¼ĘĖć£»
£Ø2£©Ķ¬ÖÜĘŚÖŠĻ”ÓŠĘųĢåµŚŅ»µēĄėÄÜ×ī“ó£¬ĘäĖüŌŖĖŲĖęŌ×ÓŠņŹż×ī“óŌŖĖŲµŚŅ»µēĄėÄܳŹŌö“óĒ÷ŹĘ£¬µ«¢ņA×唢¢õA×å×īøßÄܼ¶·Ö±šĪŖČ«Āś”¢°ėĀśĪȶØדĢ¬£¬µŚŅ»µēĄėÄÜøßÓŚĶ¬ÖÜĘŚĻąĮŚŌŖĖŲµÄ£¬¹ŹµŚ¶žÖÜĘŚÖŠµŚŅ»µēĄėÄÜĖ³ŠņĪŖ£ŗNe£¾F£¾N£¾O£¾C£¾Be£¾B£¾Li£¬XŗĶY¾łĪŖµŚ2ÖÜĘŚpĒųŌŖĖŲ£¬ĘäŌ×ӵĵŚŅ»µēĄėÄÜŹżÖµ°““ӓ󵽊”µÄĖ³ŠņŌŚĶ¬ÖÜĘŚŌŖĖŲÖŠ“¦ÓŚµŚČżĪ»ŗĶµŚĘßĪ»£¬ŌņXĪŖNŌŖĖŲ”¢YĪŖBŌŖĖŲ£»
¢ŁĆæøöČż½ĒŠĪŗ¬ÓŠ3øöYŌ×Ó£¬ĆæøöYŌ×ÓĪŖ5øöČż½ĒŠĪ¹²ÓĆ£¬ĆæøöČż½ĒŠĪŗ¬ÓŠ3øöY-Y¼ü£¬¶ųĆæøöY-Y¼üĪŖ2øöČż½ĒŠĪ¹²ÓĆ£¬ĄūÓĆ¾łĢƷؼĘĖć£»
¢ŚČō½«Ņ»øöÕż¶žŹ®ĆęĢåÖŠµÄĆæŅ»øö¶„µćĻ÷Č„£¬æÉ»ńµĆÖ»ÓÉÕżĮł±ßŠĪŗĶÕżĪå±ßŠĪ¹¹³ÉµÄ¶ąĆęĢ壮Čō¶ąĆęĢåµÄĆæŅ»øö¶„µć¾łĪŖĢ¼Ō×Ó£¬ŌņĆæøöĢ¼Ō×ÓŠĪ³É3øöĢ¼Ģ¼¦Ņ¼ü£¬ĆæøöĢ¼Ģ¼¦Ņ¼üĪŖ1øöĢ¼Ō×ÓĢį¹©$\frac{1}{2}$£¬ĄūÓĆ¾łĢƷؼĘĖć1molC60·Ö×ÓÖŠŗ¬ÓŠ¦Ņ¼üµÄŹżÄ棻
C60¾§Ģå²ÉČ”ĆęŠÄĮ¢·½×īĆܶѻż£¬ŅŌ¶„µćC60ŃŠ¾æ£¬ÓėÖ®ĻąĮŚµÄC60“¦ÓŚĆęŠÄ£¬Ćæøö¶„µćĪŖ8øö¾§°ū¹²ÓĆ£¬¶ųĆæøöĆęĪŖ2øö¾§°ū¹²ÓĆ£»
¢Ūa£®Įł·½”°ĄąŹÆÄ«”±YX¾§Ģ壬²ć¼äĪŖ·Ö×Ó¼ä×÷ÓĆĮ¦£¬×÷ÓĆĮ¦Š”£»
b£®Į¢·½”°Ąą½šøÕŹÆ”±YX¾§Ģ壬ĪŖæÕ¼äĶųד½į¹¹£¬ŹōÓŚŌ×Ó¾§Ģ壻
c£®Į¢·½”°Ąą½šøÕŹÆ”±BN¾§ĢåÖŠ£¬BŌ×ÓŠĪ³É4øöB-N¼ü£Øŗ¬ÓŠ1øöÅäĪ»¼ü£©£¬ŌӻƹģµĄŹżÄæĪŖ4£»
£Ø3£©ZĪŖµŚ4ÖÜĘŚdĒųŌŖĖŲ£¬×īøß¼ŪĪŖ+7¼Ū£¬ŌņZĪŖMnŌŖĖŲ£¬MŗĶZŹĒĶ¬ÖÜĘŚµÄŌŖĖŲ£¬¾ßÓŠĻąĶ¬µÄ×īøß»ÆŗĻ¼Ū£¬ŌņMĪŖBr£»
¢ŁZ“¦ÓŚµÄµŚĖÄÖÜĘŚµŚ¢÷B×壻
¢Ś·ĒōĒ»łŃõŹżÄæŌ½¶ą£¬ĖįŠŌŌ½Ē棻
¢ŪĶعżX-ÉäĻßŃÜÉ䏵ŃéĒų·Ö¾§Ģ唢׼¾§ĢåŗĶ·Ē¾§Ģ壮
¢Ü¾§ĢåZæÉŠĪ³ÉĢåŠÄĮ¢·½¾§°ū£¬Ģå¶Ō½ĒĻßÉĻ3øöZŌ×ÓĻąĮŚ£¬Čō¾§°ūÖŠ¾ąĄė×ī½üµÄZŌ×ÓŗĖ¼ä¾ąĄėĪŖapm£¬ŌņĢå¶Ō½ĒĻß³¤¶ČĪŖ2a pm£¬¹Ź¾§°ūĄā³¤ĪŖ$\frac{2a}{\sqrt{3}}$pm£¬øł¾Ż¾łĢƷؼĘĖć¾§°ūÖŠZŌ×ÓŹżÄ棬±ķŹ¾³ö¾§°ūÖŹĮ棬ŌŁøł¾Ż¦Ń=$\frac{m}{V}$¼ĘĖćZ¾§ĢåµÄĆÜ¶Č£®
½ā“š ½ā£ŗ£Ø1£©HeµÄŌ×ÓŠņŹżĪŖ2£¬øł¾Żø÷ÖÜĘŚČŻÄÉŌŖĖŲÖÖŹż£¬æÉÖŖµŚĘßÖÜĘŚ0×åµÄŌ×ÓŠņŹżĪŖ2+8+8+18+18+32+32=118£¬
¹Ź“š°øĪŖ£ŗ118£»
£Ø2£©Ķ¬ÖÜĘŚÖŠĻ”ÓŠĘųĢåµŚŅ»µēĄėÄÜ×ī“ó£¬ĘäĖüŌŖĖŲĖęŌ×ÓŠņŹż×ī“óŌŖĖŲµŚŅ»µēĄėÄܳŹŌö“óĒ÷ŹĘ£¬µ«¢ņA×唢¢õA×å×īøßÄܼ¶·Ö±šĪŖČ«Āś”¢°ėĀśĪȶØדĢ¬£¬µŚŅ»µēĄėÄÜøßÓŚĶ¬ÖÜĘŚĻąĮŚŌŖĖŲµÄ£¬¹ŹµŚ¶žÖÜĘŚÖŠµŚŅ»µēĄėÄÜĖ³ŠņĪŖ£ŗNe£¾F£¾N£¾O£¾C£¾Be£¾B£¾Li£¬XŗĶY¾łĪŖµŚ2ÖÜĘŚpĒųŌŖĖŲ£¬ĘäŌ×ӵĵŚŅ»µēĄėÄÜŹżÖµ°““ӓ󵽊”µÄĖ³ŠņŌŚĶ¬ÖÜĘŚŌŖĖŲÖŠ“¦ÓŚµŚČżĪ»ŗĶµŚĘßĪ»£¬ŌņXĪŖNŌŖĖŲ”¢YĪŖBŌŖĖŲ£¬£®
¢ŁĆæøöČż½ĒŠĪŗ¬ÓŠ3øöYŌ×Ó£¬ĆæøöYŌ×ÓĪŖ5øöČż½ĒŠĪ¹²ÓĆ£¬Ōņŗ¬ÓŠYŌ×ÓŹżÄæĪŖ$\frac{20”Į3}{5}$=12£¬
ĆæøöČż½ĒŠĪŗ¬ÓŠ3øöY-Y¼ü£¬¶ųĆæøöY-Y¼üĪŖ2øöČż½ĒŠĪ¹²ÓĆ£¬ŗ¬ÓŠY-Y¼üŹżÄæĪŖ$\frac{20”Į3}{2}$=30£¬
¹Ź“š°øĪŖ£ŗ12£»30£»
¢ŚČō½«Ņ»øöÕż¶žŹ®ĆęĢåÖŠµÄĆæŅ»øö¶„µćĻ÷Č„£¬æÉ»ńµĆÖ»ÓÉÕżĮł±ßŠĪŗĶÕżĪå±ßŠĪ¹¹³ÉµÄ¶ąĆęĢ壮Čō¶ąĆęĢåµÄĆæŅ»øö¶„µć¾łĪŖĢ¼Ō×Ó£¬ĆæøöĢ¼Ō×ÓĪŖ1øöĪå±ßŠĪ”¢2øöĮł±ßŠĪ¹²ÓĆ£¬ŌņĆæøöĢ¼Ō×ÓŠĪ³É3øöĢ¼Ģ¼¦Ņ¼ü£¬ĆæøöĢ¼Ģ¼¦Ņ¼üĪŖ1øöĢ¼Ō×ÓĢį¹©$\frac{1}{2}$£¬¹Ź1molC60·Ö×ÓÖŠŗ¬ÓŠ¦Ņ¼üĪŖ$\frac{60”Į3}{2}$mol=90mol£¬¼“ŗ¬ÓŠ90NA øö¦Ņ¼ü£¬
¹ŹC60¾§Ģå²ÉČ”ĆęŠÄĮ¢·½×īĆܶѻż£¬ŅŌ¶„µćC60ŃŠ¾æ£¬ÓėÖ®ĻąĮŚµÄC60“¦ÓŚĆęŠÄ£¬Ćæøö¶„µćĪŖ8øö¾§°ū¹²ÓĆ£¬¶ųĆæøöĆęĪŖ2øö¾§°ū¹²ÓĆ£¬ŌņĘäÅäĪ»ŹżĪŖ$\frac{3”Į8}{2}$=12£¬
¹Ź“š°øĪŖ£ŗ90NA£»12£»
¢Ūa£®Įł·½”°ĄąŹÆÄ«”±YX¾§Ģ壬²ć¼äĪŖ·Ö×Ó¼ä×÷ÓĆĮ¦£¬×÷ÓĆĮ¦Š”£¬ÖŹµŲČķ£¬æÉ×öøßĪĀČ󻬼Į£¬¹ŹaÕżČ·£»
b£®Į¢·½”°Ąą½šøÕŹÆ”±YX¾§Ģ壬ĪŖæÕ¼äĶųד½į¹¹£¬ŹōÓŚŌ×Ó¾§Ģ壬³¬Ó²²ÄĮĻ£¬ÓŠÓÅŅģµÄÄĶÄ„ŠŌ£¬¹ŹbÕżČ·£»
c£®Į¢·½”°Ąą½šøÕŹÆ”±BN¾§ĢåÖŠ£¬BŌ×ÓŠĪ³É4øöB-N¼ü£Øŗ¬ÓŠ1øöÅäĪ»¼ü£©£¬ŌӻƹģµĄŹżÄæĪŖ4£¬BŌ×Ó²ÉČ”sp3Ōӻƣ¬¹Źc“ķĪó£¬
¹Ź“š°øĪŖ£ŗab£»
£Ø3£©ZĪŖµŚ4ÖÜĘŚdĒųŌŖĖŲ£¬×īøß¼ŪĪŖ+7¼Ū£¬ŌņZĪŖMnŌŖĖŲ£¬MŗĶZŹĒĶ¬ÖÜĘŚµÄŌŖĖŲ£¬¾ßÓŠĻąĶ¬µÄ×īøß»ÆŗĻ¼Ū£¬ŌņMĪŖBr£»
¢ŁZ“¦ÓŚµÄµŚĖÄÖÜĘŚµŚ¢÷B×壬¼Ūµē×ÓÅŲ¼Ź½ĪŖ3d54s2£¬¹Ź“š°øĪŖ£ŗ3d54s2£»
¢ŚHMO2ÖŠ·ĒōĒ»łŃõŌ×ÓŹżÄæ±ČHMOµÄ¶ą£¬¹ŹĖįŠŌHMO£¼HMO2£¬¹Ź“š°øĪŖ£ŗ£¼£»HMO2ÖŠ·ĒōĒ»łŃõŌ×ÓŹżÄæ±ČHMOµÄ¶ą£»
¢ŪĶعżX-ÉäĻßŃÜÉ䏵ŃéĒų·Ö¾§Ģ唢׼¾§ĢåŗĶ·Ē¾§Ģ壬¹Ź“š°øĪŖ£ŗX-ÉäĻßŃÜÉ䏵Ń飻
¢Ü¾§ĢåZæÉŠĪ³ÉĢåŠÄĮ¢·½¾§°ū£¬Ģå¶Ō½ĒĻßÉĻ3øöZŌ×ÓĻąĮŚ£¬Čō¾§°ūÖŠ¾ąĄė×ī½üµÄZŌ×ÓŗĖ¼ä¾ąĄėĪŖa pm£¬ŌņĢå¶Ō½ĒĻß³¤¶ČĪŖ2a pm£¬¹Ź¾§°ūĄā³¤ĪŖ$\frac{2a}{\sqrt{3}}$pm£¬¾§°ūÖŠMnŌ×ÓŹżÄæĪŖ8”Į$\frac{1}{8}$+1=2£¬Ōņ¾§°ūÖŹĮæĪŖ2”Į$\frac{55}{{N}_{A}}$g£¬¹Ź¾§ĢåµÄĆܶČĪŖ2”Į$\frac{55}{{N}_{A}}$g”Ā£Ø$\frac{2a}{\sqrt{3}}$”Į10-10 cm£©3=$\frac{165\sqrt{3}”Į1{0}^{30}}{4{a}^{3}{N}_{A}}$g•cm-3£¬
¹Ź“š°øĪŖ£ŗ$\frac{165\sqrt{3}”Į1{0}^{30}}{4{a}^{3}{N}_{A}}$£®
µćĘĄ ±¾ĢāŹĒ¶ŌĪļÖŹ½į¹¹ÓėŠŌÖŹµÄ漲飬Éę¼°ŌŖĖŲÖÜĘŚ±ķ”¢µēĄėÄÜ”¢ŗĖĶāµē×ÓÅŲ¼”¢ŌӻƷ½Ź½”¢¾§ĢåĄąŠĶÓėŠŌÖŹ”¢¾§°ū¼ĘĖćµČ£¬²ąÖŲ¾§°ū¼ĘĖćµÄ漲飬ŠčŅŖѧɜ¾ß±øŅ»¶ØµÄæÕ¼äĻėĻóÓėŹżŃ§¼ĘĖćÄÜĮ¦£¬ÄŃ¶Č½Ļ“ó£¬ŹōÓŚŅדķĢāÄ森


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ${\;}_{1}^{1}$HŗĶ${\;}_{1}^{3}$H | B£® | ¶”ĶéŗĶŅģ¶”Ķé | C£® | O2ŗĶO3 | D£® | H2OŗĶH2O2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
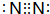 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
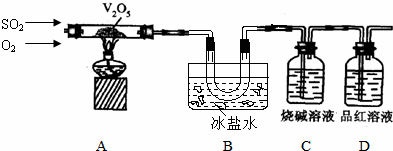
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
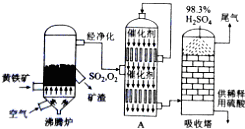
| ·ŠĢŚĀÆĪĀ¶Č/”ę | 600 | 620 | 640 | 660 |
| ĀÆŌüÖŠCuSO4µÄÖŹĮæ·ÖŹż/% | 9.3 | 9.2 | 9.0 | 8.4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
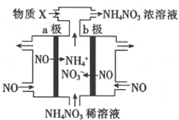 µē½āNOÖʱøNH4NO3µÄ¹¤×÷ŌĄķČēĶ¼ĖłŹ¾£¬ĪŖŹ¹µē½ā²śĪļČ«²æ×Ŗ»ÆĪŖNH4NO3£¬ĶØČėĪļÖŹX£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
µē½āNOÖʱøNH4NO3µÄ¹¤×÷ŌĄķČēĶ¼ĖłŹ¾£¬ĪŖŹ¹µē½ā²śĪļČ«²æ×Ŗ»ÆĪŖNH4NO3£¬ĶØČėĪļÖŹX£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | a¼«½ÓµēŌ“µÄøŗ¼« | |
| B£® | Ńō¼«·“Ó¦ĪŖ£ŗNO-3e-+2H2O=NO3-+4H+ | |
| C£® | Ņõ¼«·“Ó¦ĪŖ£ŗNO+5e-+6H+=NH4++H2O | |
| D£® | XĪŖNH3×īÖÕÖʵĆ3molNH4NO3ĄķĀŪÉĻŠč²¹³ä2molXĪļÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
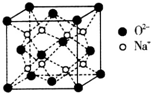 H”¢C”¢N”¢O”¢Na”¢Fe”¢CuŹĒ³£¼ūµÄĘßÖÖŌŖĖŲ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
H”¢C”¢N”¢O”¢Na”¢Fe”¢CuŹĒ³£¼ūµÄĘßÖÖŌŖĖŲ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ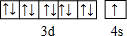 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
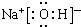 £®
£®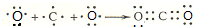
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ×īĶā²ćµē×ÓŹż£ŗY£¾Z | |
| B£® | ŗĖµēŗÉŹż£ŗa£¾b | |
| C£® | ĪČ¶ØŠŌ£ŗH2Y£¾HZ | |
| D£® | Xµ„ÖŹŅ»¶ØÄÜ“ÓWµÄŃĪČÜŅŗÖŠÖĆ»»³öWµ„ÖŹ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com