
 ��
������ Ԫ��Xλ�ڵ������ڣ����̬ԭ����4��δ�ɶԵ��ӣ���������Ų�ʽΪ1s22s22p63s23p63d64s2����XΪFe��Yԭ�����������������ڲ����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����YΪOԪ�أ�Ԫ��Z��̬ԭ�ӵ�3p�������4�����ӣ���������Ų�ʽΪ1s22s22p63s23p4����ZΪSԪ�أ�Wԭ�ӵ�2p�������3��δ�ɶԵ��ӣ���������Ų�ʽΪ1s22s22p3����WΪNԪ�أ�
��� �⣺Ԫ��Xλ�ڵ������ڣ����̬ԭ����4��δ�ɶԵ��ӣ���������Ų�ʽΪ1s22s22p63s23p63d64s2����XΪFe��Yԭ�����������������ڲ����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����YΪOԪ�أ�Ԫ��Z��̬ԭ�ӵ�3p�������4�����ӣ���������Ų�ʽΪ1s22s22p63s23p4����ZΪSԪ�أ�Wԭ�ӵ�2p�������3��δ�ɶԵ��ӣ���������Ų�ʽΪ1s22s22p3����WΪNԪ�أ�
��1����ͬ�������϶��µ縺�Լ�С���ʵ縺��O��S��
�ʴ�Ϊ��O��
������SO32-��Sԭ�ӹµ��Ӷ���=$\frac{6+2-2��3}{2}$=1���۲���Ӷ���=3+1=4���ռ�ṹΪ�����Σ�Sԭ�Ӳ�ȡsp3�ӻ���
�ʴ�Ϊ�������Σ�sp3��
��SO2������Sԭ�ӹµ��Ӷ���=$\frac{6-2��2}{2}$=1���۲���Ӷ���=2+1=3���ռ�ṹΪV�Σ�Sԭ�Ӳ�ȡsp2�ӻ�������������������IJ��غϣ�Ϊ���Է��ӣ�
�ʴ�Ϊ��V�Σ�sp2�����ԣ�
��NԪ��ԭ��2p���Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ�����Ԫ�صģ�
�ʴ�Ϊ��N��
��2��H2O�������Ҵ����Ӽ���γ��������H2S���ܣ�H2O���Ҵ��е��ܽ�ȴ���H2S��
�ʴ�Ϊ��H2O�������Ҵ����Ӽ���γ��������H2S���ܣ�
��3���ٻ�̬Fe2+�ĵ����Ų�ʽ�ǣ�1s22s22p63s23p63d6��
�ʴ�Ϊ��1s22s22p63s23p63d6��
�ڰ�����������3���Ҽ���CN-�к���1���Ҽ����γ�6����λ����Ҳ���ڦҼ���1mol�����K3[Fe��CN��5��NH3��]�к�14mol�Ҽ���
�ʴ�Ϊ��14mol��
��Feԭ�ӵļ۵����Ų�ͼΪ�� ��
��
�ʴ�Ϊ�� ��
��
��[X��CN��5��NH3��]3-��������Fe2+��CN-��NH3�γ���λ����CN-��NH3�д��ڼ��Թ��ۼ���û����������Ӽ���
�ʴ�Ϊ��ac��
���� ���⿼���Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����縺�ԡ������ܡ��ռ乹�����ӻ���ʽ�жϡ��������ѧ���ȣ�ע��ͬ���ڵ�һ�������쳣ԭ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��Na+��HCO${\;}_{3}^{-}$��Cl- | B�� | Fe3+��SCN-��Cl-��SO${\;}_{4}^{2-}$ | ||
| C�� | NH${\;}_{4}^{+}$��Fe2+��SO${\;}_{4}^{2-}$��NO${\;}_{3}^{-}$ | D�� | Mg2+��Fe2+��SO${\;}_{4}^{2-}$��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
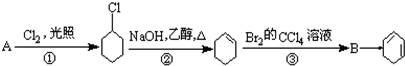
 ��B��������1��2-���廷���飮
��B��������1��2-���廷���飮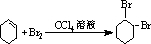 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
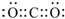 ��
��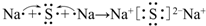 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
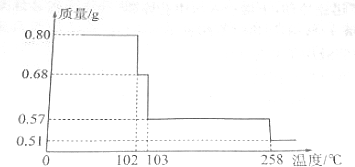
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
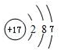 �����������Ļ�ѧʽΪ��Cl2O7��
�����������Ļ�ѧʽΪ��Cl2O7��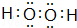 ��
��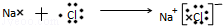 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com