
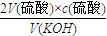 ������
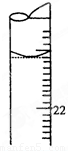
������ ��֪���ⶨKOH��Ũ��ƫ�ߣ�
��֪���ⶨKOH��Ũ��ƫ�ߣ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
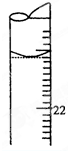 ij����������Ʒ�к����������������õ����ʣ�Ϊ�˲ⶨ�������������������µζ�������
ij����������Ʒ�к����������������õ����ʣ�Ϊ�˲ⶨ�������������������µζ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���㽭ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�ӵ�ʳ���к��еĵ������һ�ְ�ɫ�ᾧ��ĩ�������º��ȶ���������560�濪ʼ�ֽ⡣�����������µ������һ�ֽ�ǿ��������������⻯��������εȻ�ԭ�����ʷ�Ӧ����ҵ��������ص��������£�

��1����������������ص���Ҫ������ ��
��2�������±�����ص��ܽ�ȣ������۵õ�����ؾ��壬�ɾ��� �����ˡ�ϴ�ӡ�����Ȳ��衣
|
�¶�/�� |
20 |
40 |
60 |
80 |
|
KIO3/100gˮ |
8.08 |
12.6 |
18.3 |
24.8 |
��3����֪��KIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O�� I2+2S2O32-=2I-+S4O62-
Ϊ�˲ⶨ�ӵ�ʳ���е�ĺ�����ijѧ�����������ʵ�飺ȷ��ȡwgʳ�Σ�����������ˮʹ����ȫ�ܽ⣻����ϡ�����ữ������Һ���������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��������ָʾ���������ʵ���Ũ��Ϊ2.00��10-3mol��L-1��Na2S2O3��Һ�ζ�������10.00mLʱǡ�÷�Ӧ��ȫ��
�ڵζ������У����õIJ�������Ϊ �� ��
��ʵ������� ��ָʾ�����ζ��յ�������� ��
�� �����йظõζ�ʵ���˵������ȷ���� ��
A���ζ�����ʹ��ǰ�������Ƿ�©ˮ����ϴ B���ζ�ʱ�۾�ע�ӵζ�������ҺҺ��仯
C��Ϊ��Сʵ������������������ˮ��ϴ��ƿ�ڱ� D���յ����ʱ���ӻᵼ�µ�ĺ���ƫ��
�ܼӵ�ʳ����Ʒ�еĵ�Ԫ�غ����� g��kg-1���Ժ�w�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
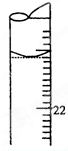 ij����������Ʒ�к����������������õ����ʣ�Ϊ�˲ⶨ�������������������µζ�������
ij����������Ʒ�к����������������õ����ʣ�Ϊ�˲ⶨ�������������������µζ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
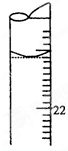
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com