
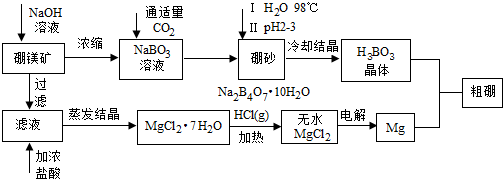
���� ��þ����Ҫ�ɷ�ΪMg2B2O5•H2O����ɰ�Ļ�ѧʽΪNa2B4O7•10H2O��������þ����ȡ����þ������Ĺ�����������þ�������������Ũ��Һ���˵õ��Ȼ�þ������Ũ�����ܽ�ͨ������Ũ���õ��Ȼ�þ�ᾧˮ������Ȼ��������м��ȵõ��Ȼ�þ���壬���õ�þ����Һ����Ҫ��NaBO2��ͨ������������̼����õ���ɰ��������ˮ����H2SO4��pH2��3��ȡH3BO3�����ȵõ�B2O3��
��1����ɰ�Ļ�ѧʽΪNa2B4O7•10H2O������Ԫ�ػ��ϼ۱�ע������Ԫ�ػ��ϼۣ���H2SO4��pH2��3����ɰ�е�Na2B4O7������Һ������H3BO3 ��
��2���Ȼ�þ��ˮ��Һ��ˮ������������þ�����Ե缫���MgCl2��Һ���������ӵõ���������������ˮ�ĵ���ƽ���ƻ���ˮ������������������Ũ������þ�����γ�������þ�������ݴ���д���ӷ��̣�
��3��BI2�е�ϵͣ��ᴿ����������е������������ķ�������õ������ɵĵ���0.3mol/L��Na2S2O3��Һ�ζ����յ㣬��Ҫ������Һ����Ӧ��ָʾ����H2S2O3Ϊ���ᣬNa2S2O3��Һ�Լ��ԣ��ݴ�ѡ��ζ��ܣ����ݹ�ϵʽB��BI3��$\frac{3}{2}$I2��3S2O32-���ζ����ݼ������������ĺ�����
��4��ȼ�ϵ�����������ǹ�������õ���������ˮ�����ݵ������ҺPH�仯��ϵ�ط�Ӧ����þ����Ũ�ȣ�PH=6��������������Ũ�ȣ��������þ����Ũ�ȼ���Ũ���̺��ܶȻ������ȽϷ����Ƿ�����������þ������
��� �⣺��1����ɰ�Ļ�ѧʽΪNa2B4O7•10H2O����Ԫ�ػ��ϼ�Ϊ+1�ۣ���Ԫ�ػ��ϼ�-2�ۣ����ݻ��ϼ۴����ͼ���õ���Ԫ�ػ��ϼ�Ϊ+3�ۣ���H2SO4��pH2��3����ɰ�е�Na2B4O7������Һ������H3BO3 ����Ӧ�����ӷ���ʽΪ��B4O72-+2H++5H2O=4H3BO3��
�ʴ�Ϊ��+3��B4O72-+2H++5H2O=4H3BO3��
��2��MgCl2•7H2O��Ҫ��HCl��Χ�м��ȣ���Ϊ�˷�ֹ�Ȼ�þˮ������������þ�����ö��Ե缫���MgCl2��Һ�����������ӵõ���������������ˮ�ĵ���ƽ���ƻ���ˮ������������������Ũ������þ�����γ�������þ��������Ӧ�����ӷ���ʽΪ2H2O+Mg2++2Cl-$\frac{\underline{\;ͨ��\;}}{\;}$H2��+Mg��OH��2��+Cl2����
�ʴ�Ϊ����ֹMgCl2ˮ������Mg��OH��2��2H2O+Mg2++2Cl-$\frac{\underline{\;ͨ��\;}}{\;}$H2��+Mg��OH��2��+Cl2����
��3���ƵõĴ������һ��������I2��������BI2�����ᴿBI2��BI2�е�ϵͣ��ᴿ�ɲ�������ķ�������õ����ʴ�Ϊ������
�����ɵĵ���0.3mol/L��Na2S2O3��Һ�ζ����յ㣬��Ҫ������Һ����Ӧ��ָʾ�����ʴ�Ϊ��������Һ��
�������������Һ�ʼ��ԣ�ѡ���ʽ�ζ��ܣ��ʴ�Ϊ����ʽ��
����������Ƶ����ʵ���Ϊ��0.30mol/L��0.036L=0.0108mol�����ݹ�ϵʽ��B��BI3��$\frac{3}{2}$I2��3S2O32-��n��B��=$\frac{1}{3}$n��S2O32-��=0.0108mol��$\frac{1}{3}$=0.0036mol��
�������Ϊ��11g/mol��0.0036mol=0.0396g����������ĺ���Ϊ��$\frac{0.0396g}{0.04g}$��100%=99%��
�ʴ�Ϊ��99%��
��4��þ-H2O2����ȼ�ϵ�صķ�Ӧ����ΪMg+H2O2+2H+�TMg2++2H2O���������ǹ�������õ���������ˮ�ķ�Ӧ��������ӦʽH2O2+2H++2e-=2H2O������ʼ�������ҺpH=1����pH=2ʱ��Һ�У�������Ũ�ȼ�С0.1mol/L-0.01mol/L=0.09mol/L�����ݷ�Ӧ����ʽ�õ�Mg2+����Ũ��=0.045mol/L��Ksp[Mg��OH��2]=5.6��10-12������ҺpH=6ʱ��c��OH-��=10-8mol/L����Qc=c��Mg2+����c2��OH-��=0.045mol/L��10-16mol/L=4.5��10-18��Ksp[Mg��OH��2]��˵����������þ�������ɣ�ֻ��Mg2+��ʽ���ڣ�
�ʴ�Ϊ��H2O2+2H++2e-=2H2O��0.045 mol•L-1��Mg2+��ʽ���ڣ�û��Mg��OH��2������
���� ���⿼���������ˮ�⡢ԭ���ԭ���͵���ԭ���ķ����������ܽ�ƽ��ļ���Ӧ�ã���Ŀ�Ѷ��Դ������ѵ㣬����ʱҪ���������Ŀ����������ϵ�ͱ������ݣ�����������ϵ�����������ĸ�����ٽ��⣮


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
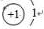 ��C�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬�ӽṹ�Ƕ��ж�A2Ӧ��C2���� �����ȶ�����ã������ǵ��������ļ��ܴ���H-H
��C�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬�ӽṹ�Ƕ��ж�A2Ӧ��C2���� �����ȶ�����ã������ǵ��������ļ��ܴ���H-H �����к��еĻ�ѧ�����������Ӽ����ۼ�
�����к��еĻ�ѧ�����������Ӽ����ۼ�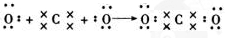 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2.24L CO2�к��е�ԭ����Ϊ0.3NA | B�� | 20g D2O������������Ϊ10NA | ||
| C�� | 16g CH4�к�C-H����ĿΪNA | D�� | 1 mol �������е�ԭ����Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

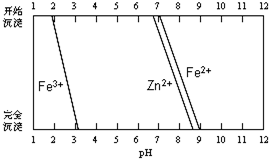
| �������� | Fe��OH��3 | Fe��OH��2 |
| ��ʼ������pH | 1.5 | 6.5 |
| ������ȫ��pH | 3.7 | 9.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |
| B�� |
| C�� |
| D�� |
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaCl2 | B�� | Na2O | C�� | H2SO4 | D�� | NH4Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
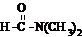 ����ˮ
����ˮ �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com